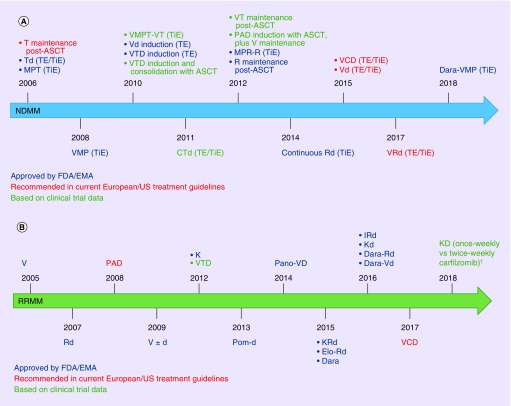
Figure 1. . Timeline of key agents and treatment combinations that are approved/recommended or have been investigated in Phase III clinical trials.
In the (A) NDMM and (B) RRMM settings.
Not all regimens or agents are approved or available in all countries.
†Carfilzomib twice-weekly (20/27 mg/m2) versus once-weekly (20/70 mg/m2).
ASCT: Autologous stem cell transplant; CTd: Cyclophosphamide, thalidomide, dexamethasone; d: Dexamethasone; Dara: Daratumumab; Dara-Rd: Daratumumab, lenalidomide, dexamethasone; Dara-Vd: Daratumumab, bortezomib, dexamethasone; Dara-VMP: Daratumumab, bortezomib, melphalan, prednisone; Elo-Rd: Elotuzumab, lenalidomide, dexamethasone; EMA: European Medicines Agency; EMN: European Myeloma Network; ESMO: European Society for Medical Oncology; IRd: Ixazomib, lenalidomide, dexamethasone; K: Carfilzomib; Kd/D, carfilzomib, dexamethasone; KRd: Carfilzomib, lenalidomide, dexamethasone; MPR-R: Melphalan, prednisone, lenalidomide, plus lenalidomide maintenance; MPT: Melphalan, prednisone, thalidomide; NDMM: Newly diagnosed multiple myeloma; PAD: Bortezomib, doxorubicin, dexamethasone; Pano-VD: Panobinostat, bortezomib, dexamethasone; Pom-d: Pomalidomide, dexamethasone; R: Lenalidomide; Rd: Lenalidomide, dexamethasone; RRMM: Relapsed/refractory multiple myeloma; T: Thalidomide; Td: Thalidomide, dexamethasone; TE: Transplant-eligible; TiE: Transplant-ineligible; V: Bortezomib; VCD: Bortezomib, cyclophosphamide, dexamethasone; Vd: Bortezomib, dexamethasone; VMP: Bortezomib, melphalan, prednisone; VMPT: Bortezomib, melphalan, prednisone, thalidomide; VRd: Bortezomib, lenalidomide, dexamethasone; VT: Bortezomib, thalidomide; VTD: Bortezomib, thalidomide, dexamethasone.