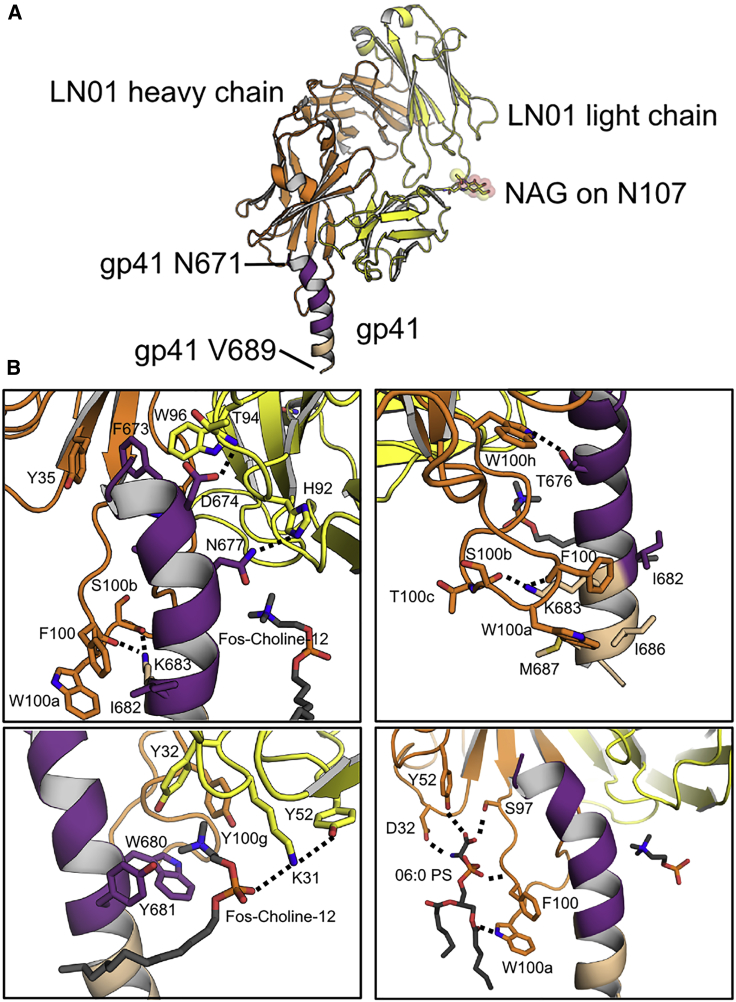
Figure 5.
Structure of LN01 in Complex with gp41 MPER-TM1 and with Lipid
(A) Structure of the LN01-MPER-TM1 complex. LN01 is colored in yellow (light chain) and orange (heavy chain) and the N-acetyl-β-d-glucosamine (NAG) on Asn107 of the light chain is shown with spheres. Gp41 is colored in purple (MPER) and beige (TM). The same coloring was used in all figures.
(B) Close up of the interactions with gp41 MPER, Fos-Choline-12, and PS revealing two lipid-binding sites on either side of the MPER helix. Fos-Choline-12, PS, and residues involved in polar contacts and important hydrophobic contacts are indicated and represented in sticks; hydrogen bond interactions are represented as dashed lines. The upper two panels show the interactions of LN01 with gp41 MPER from two different orientations. The lower left panel shows the interaction with Fos-Choline-12, a putative lipid-binding site accommodating phosphatidylcholine, and the lower right panel shows the hydrogen bond network that coordinates 06:0 PS forming a second lipid-binding pocket.