Fig. 1.
Models to study collective cell migrationin vivoand their microenvronments.
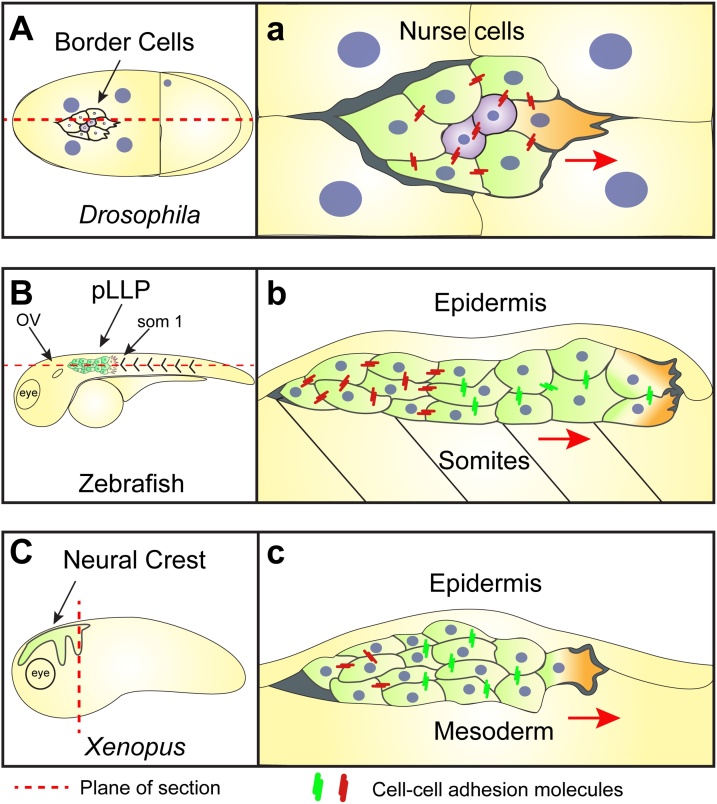
A, a. Schematics of Drosophila border cell anatomical position and collective migration. (A) Border cell migration in a Drosophila embryo. (a) Border cells migrate in a confined space, surrounded by gigantic nurse cells. High levels of AJs proteins are required to resist deformation and for the mechanical feedback require for directional collective migration. B, b. Diagrams showing topographic location and confined collective migration of the posterior lateral line primordia (pLLP). (B) Initial position of the pLLP among the otic vesicle (OV) and the first somite (som 1). (b) Sagittal section showing how the pLLP migrates in a confined space between the somatic mesoderm and epidermis. Differential antero-posterior distribution of AJs is observed, while E-cadherin localises to the rear, the leading edge express a more dynamic N-cadherin. C, c. Drawings representing anatomical position and confinement during neural crest collective migration. (C) In Xenopus, cephalic neural crest migrates as a collective from dorsal towards ventral and anterior territories in well-defined streams. (c) Diagram represent a transverse section across the head of a Xenopus embryo and it shows the high degree of confinement experienced by the neural crest while migrating ‘sandwiched’ between the epidermis and underlying head mesoderm. Neural crest relies mostly in N-cadherin to mediate transient contacts require for the coordinated collective migration. Red arrows show the direction of migration for each tissue.