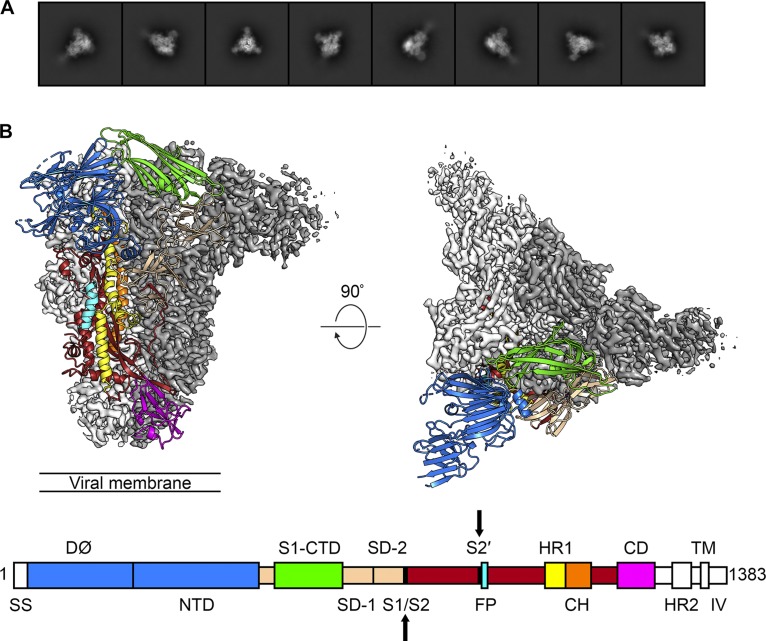
FIG 1.
The 3.1-Å structure of PEDV S in the prefusion conformation. (A) 2D class averages determined for the PEDV S protein. (B) The densities for two protomers are shown, colored white and gray. The third protomer is shown as ribbons, colored by domain corresponding to the primary structure diagram. SS, signal sequence; S1/S2, S1/S2 protease cleavage site; S2′, S2′ protease cleavage site; FP, fusion peptide; HR1, heptad repeat 1; CH, central helix; CD, connector domain; HR2, heptad repeat 2; TM, transmembrane domain; IV, intravirion tail. Protease cleavage sites S1/S2 and S2′ are highlighted by arrows on the primary structure diagram.