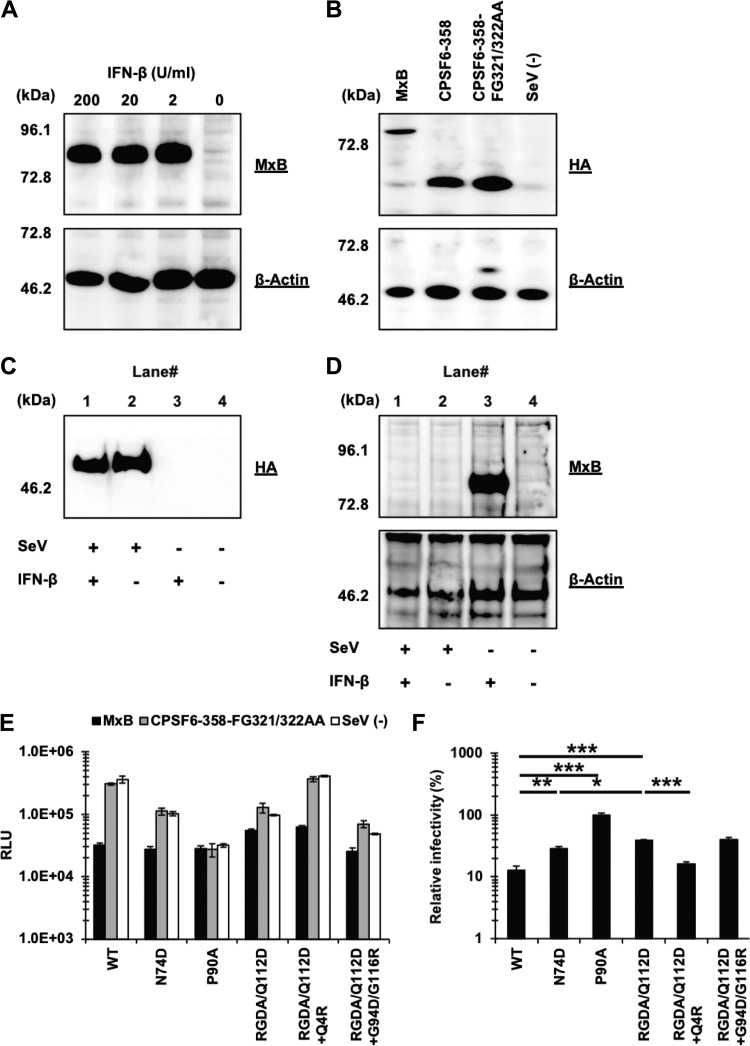
FIG 6.
The Q4R mutation sensitizes the RGDA/Q112D virus to MxB restriction despite conferring IFN-β resistance. (A) Expression level of MxB in Jurkat cells treated with 200, 20, 2, or 0 U per ml of IFN-β. Western blots of cell lysates extracted from IFN-β-treated cells were probed with an anti-MxB antibody (top) or an anti-β-actin antibody (bottom). The positions of the molecular weight markers are shown on the left side. (B) The expression level of HA-tagged MxB, CPSF6-358, and CPSF6-358-FG321/322AA (control) in SeV-infected MT4 cells was evaluated using a rat anti-HA monoclonal antibody (top). The membrane was reprobed with an anti-β-actin antibody (bottom). The positions of the molecular weight markers are shown on the left side. (C) The expression level of HA-tagged CPSF6-358-FG321/322AA in SeV-infected MT4 cells was evaluated using a rat anti-HA monoclonal antibody. The position of the molecular weight marker is shown on the left side. (D) Expression level of MxB in MT4 cells infected with SeV expressing CPSF6-358-FG321/322AA in the presence of either 0 or 200 per ml IFN-β. Cell lysates were probed with an anti-MxB antibody (top) or an anti-β-actin antibody (bottom). The positions of the molecular weight markers are shown on the left side. (E) MT4 cells expressing MxB or CPSF6-358-FG321/322AA or SeV− cells were superinfected with reverse transcriptase-normalized VSV-G-pseudotyped HIV-1 isolates encoding the luciferase reporter gene. The RLU were determined at 2 days after infection. One representative result of at least three independent experiments is shown, with error bars denoting the standard deviation (SD) of the mean of triplicate measurements. (F) The degree of sensitivity to MxB was calculated by dividing the RLU of each virus in the presence of MxB by those in the presence of CPSF6-358-FG321/322AA (control). The mean from three independent experiments is shown, with error bars denoting the standard error of the mean (SEM). ***, P < 0.001; **, P < 0.01; *, P < 0.05.