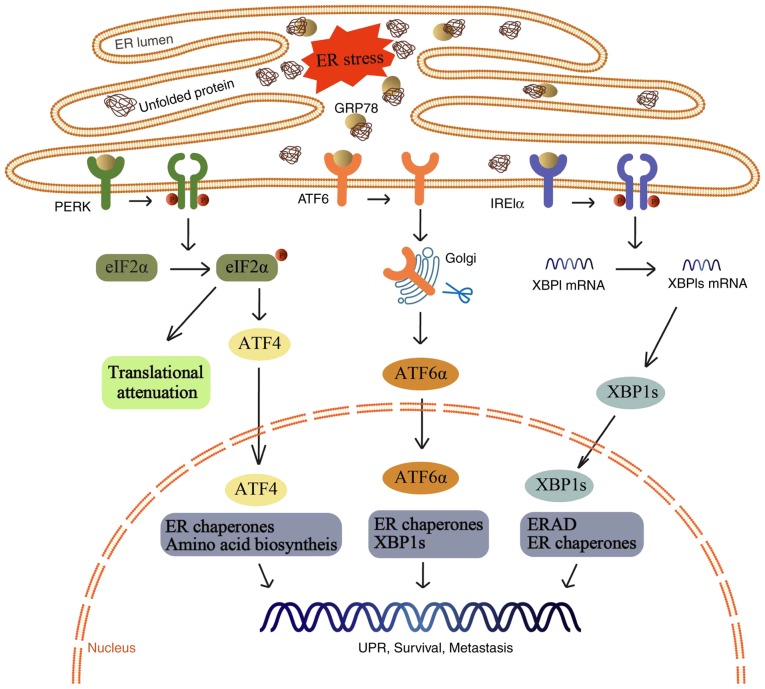
Figure 1.
A variety of physiological conditions can disrupt the protein folding process and consequently result in the accumulation of unfolded and misfolded proteins in the ER, a condition termed ER stress. Under ER stress, the UPR is activated to minimize overloading. If the UPR is successful in reducing the number of unfolded and misfolded proteins, UPR signaling is attenuated and the cell survives. ER, endoplasmic reticulum; GRP78, 78-kDa glucose-regulated protein; PERK, protein kinase R-like ER kinase; eIF2α, eukaryotic translation initiation factor 2α; ATF4, activating transcription factor 4; ATF6, activating transcription factor 6; IRE1α, inositol-requiring enzyme 1α; XBP1, X-box binding protein 1; ERAD, endoplasmic reticulum associated degradation; UPR, unfolded protein response.