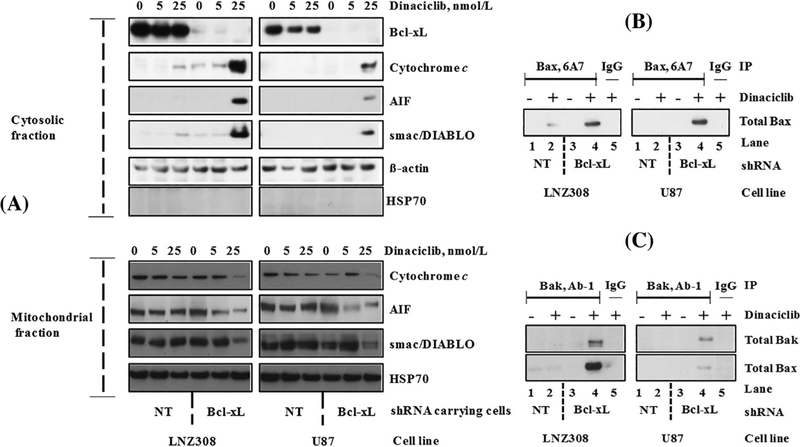
FIGURE 3.
Dinaciclib induces Bax and Bak conformational changes, and the release of caspase activators in Bcl-xL silenced cells. (A) Bcl-xL depleted (Bcl-xL shRNA) or non-target shRNA (NT) carrying LNZ308 and U87 cells were seeded at 60% confluence, allowed to attach overnight, and treated with indicated concentrations of dinaciclib for 24 h. Cytosolic (upper panel) and mitochondrial (lower panel) fractions were prepared, and equal amounts of protein were separated by SDS-PAGE and subjected to Western blotting analysis with the indicated antibodies. Lack of HSP70 in the cytosolic fraction clearly demonstrates that the cytoplasmic cytochrome c, AIF, or smac/DIABLO did not result from the mitochondrial damage during the extraction process. (B) Bcl-xL depleted (Bcl-xL shRNA) or non-target shRNA (NT) expressing LNZ308 or U87 cells were treated with or without dinaciclib (25 nmol/L) for 24 h. An equal amount of protein was immunoprecipitated (IP) with monoclonal anti-Bax (6A7; Sigma-Aldrich) antibody and then immunoblotted with polyclonal anti-Bax antibody (Cell Signaling Technology). Control IgG (Santa Cruz #2025) served as negative control (lane 5). (C) Bcl-xL depleted or non-target shRNA (NT) expressing LNZ308 or U87 cells were treated with or without dinaciclib (25 nmol/L) for 24 h. An equal amount of protein was immunoprecipitated (IP) with monoclonal anti-Bak (Ab-1; EMD Millipore) antibody and then immunoblotted with polyclonal anti-Bak antibody (Cell Signaling Technology) or with polyclonal anti-Bax antibody (Cell Signaling Technology). Dinaciclib induces Bax (B), Bak activation (C, upper panel), and Bax/Bak heterodimer formation (C, lower panel) in Bcl-xL silenced cells (compare lane 2 vs lane 4). Control IgG (Santa Cruz #2025) served as negative control (lane 5). The results of a representative study are shown; three additional experiments produced similar results