Summary:
Objective:
We aimed to determine the frequency of probable obstructive sleep apnea (pOSA) in refractory epilepsy monitoring unit inpatients and clinical features associated with pOSA, including risk for Sudden Unexpected Death in Epilepsy (SUDEP).
Methods:
We prospectively recruited 49 consecutive adult patients admitted to the Mayo Clinic Epilepsy Monitoring Unit with focal, generalized or unclassified epilepsy syndromes. pOSA was identified using oximetric oxyhemoglobin desaturation index (ODI) and the Sleep Apnea-Sleep Disorders Questionnaire (SA-SDQ) and STOP-BAG screening tools. Revised SUDEP-7 (rSUDEP-7) risk inventory scores were calculated, and epilepsy patients with and without pOSA were compared with Wilcoxon signed rank tests. Correlation and regression analyses were utilized to determine relationships between pOSA and rSUDEP-7 scores.
Results:
Thirty-five percent of patients had pOSA with a mean ODI of 11.3 ± 5.1/hour (range 5.1–22.8). Patients with pOSA were older, heavier, and more frequently had a focal epilepsy syndrome and longer epilepsy duration, with higher SA-SDQ and STOP-BAG scores (all p<0.05). Median rSUDEP-7 score was 3 ± 1.4 (0–6). Higher rSUDEP-7 scores were positively correlated with higher ODI (p=0.036). rSUDEP-7 score ≥ 5 was associated with pOSA by ODI, SA-SDQ, and STOP-BAG questionnaire criteria (p<0.05).
Significance:
Our pilot study identified a high frequency of probable OSA in refractory epilepsy monitoring patients, finding that probable OSA patients were older, heavier, with higher screening symptoms for sleep apnea and more frequent focal seizures with a longer epilepsy duration. We also found a possible association between OSA and SUDEP risk. Identification and treatment of OSA in patients with epilepsy could conceivably provide a novel approach toward preventing the risk of SUDEP. Future studies with polysomnography are needed to confirm predictive features for OSA in epilepsy populations, and to determine whether OSA is associated with SUDEP risk.
Keywords: Epilepsy, OSA, sudden death in epilepsy, SUDEP, SUDEP-7, Revised SUDEP-7
Introduction:
People with epilepsy have a twenty-fold heightened risk for sudden death relative to the general population.1 Sudden unexpected death in epilepsy (SUDEP) is the main cause of potentially avoidable, epilepsy-related deaths. SUDEP is defined as sudden, unexpected and non-traumatic death in patients with epilepsy in whom autopsy does not reveal a structural or toxic cause for death.2 SUDEP may occur with or without evidence of a preceding seizure, with exclusion of documented status epilepticus. Estimates of SUDEP incidence range from 0.09 to 9.3 per 1000 persons.3 However, in those with refractory epilepsy, SUDEP yields an estimated 35% lifetime risk.4 Mechanisms underlying SUDEP deaths remain largely unknown, although several risk factors have been identified.5 While it has not yet been validated in large scale epilepsy populations, the SUDEP-7 Inventory has been proposed to quantify SUDEP risk in living people with epilepsy based upon five factors: frequency of generalized tonic-clonic seizures (GTCS), monthly seizure frequency, number of anti-epileptic drugs (AEDs) in use, duration of epilepsy, and intellectual disability. Each risk factor is assigned a point score based on its respective odds ratio for SUDEP, with a possible score of 0–12.6 The revised SUDEP-7 (rSUDEP) inventory combines GTCS and seizure frequency measures to reduce score inflation (Table 1).7
Table 1:
Comparing the SUDEP-7 Inventory to the Revised SUDEP-7 Inventory.
| Risk Factor | Odds Ratio | SUDEP-7 | Revised SUDEP-7 |
|---|---|---|---|
| GCTS in the last 12 months |
2.4 8.1 |
0 seizures → 0
points 1–3 seizures → 1 point ≥ 4 seizures → 3 points |
0 seizures → 0
points 1–3 seizures → 1 point ≥ 4 seizures → 2 points |
| Any seizure frequency per month in the last 12 months |
2.2, 3.8, 4.6* 11.5 |
0 seizures → 0
points 1–49 seizures → 1 point > 50 seizures → 3 points |
0 seizures → 0
points 1–49 seizures → 1 point > 50 seizures → 2 points |
| Epilepsy duration |
13.9 |
0 – 29 years →
0 points ≥ 30 years → 3 points |
|
| Number of AEDs |
4.0 |
0 – 2 drugs → 0
points ≥ 3 drugs → 1 point |
|
| Cognitive Impairment |
5.0 |
IQ ≥ 70 → 0
points IQ < 70 → 2 points |
|
| Total Score | 12 points | 10 points | |
Multiple odds ratio reflect the average frequencies of any seizures in the last month over a 12-month period: ≤1, 1 to ≤15, 16 to ≤50 seizures
SUDEP is believed to most often occur in the peri-ictal state; however, its pathophysiology remains to be elucidated. The major mechanisms proposed to underlie SUDEP are cardiac, respiratory, or cerebral dysfunction, or a combination of overlapping contributions from these pathways.8 Previous research has demonstrated associations between elevated SUDEP-7 score and candidate contributors to SUDEP pathophysiology including cerebral hypoxemia,9 reduced heart rate variability,6 and post-ictal generalized EEG suppression (PGES).10 In cases of witnessed SUDEP, the consistent predominant fatal pattern was a GTC followed by a period of centrally-mediated respiratory and cardiac dysfunction, then followed variably by either cardiorespiratory arrest and death, or a terminal brief partial restoration of cardiac rhythm prior to terminal apnea and cardiac arrest. Terminal apnea superseded asystole, and 87.5% of SUDEP cases occurred during sleep, suggesting potential roles for either a sleep state-dependent influence upon SUDEP risk, and/or contributions of sleep disorders such as sleep apnea to SUDEP pathophysiology.11 Further, magnetic resonance imaging of patients at high risk for SUDEP and those who suffered SUDEP have shown an enlarged right anterior hippocampus, amygdala and parahippocampus and reduced posterior thalamic grey matter volume12 These findings provide further evidence for autonomic dysfunction underlying SUDEP due to their roles in regulating sympathetic outflow to the medulla and respiratory variability in response to hypoxia, respectively.
Obstructive sleep apnea (OSA) has been previously identified as an independent risk factor for sudden cardiac death13, 14 and is linked to cardiovascular disease, arrhythmia (including bradyarrhythmia and asystole), and increased mortality.15;16 The frequency of co-morbid OSA in various epilepsy cohorts has been estimated to range between approximately 10–30%, and is more frequent in those with refractory epilepsy.17–20 While it is unclear whether the association between SCD and OSA is relevant to the likely distinct neurocardiorespiratory factors and events thought to mediate SUDEP, there are parallels between these entities in that both SCD and refractory epilepsy have a distinctive link to sleep, implying that OSA might promote sudden death in either case, or alternatively, that OSA may potentiate underlying risk factors and pathophysiologic factors underlying SUDEP. We aimed to determine the frequency of co-morbid OSA and utility of screening instruments for detecting OSA in our refractory monitored epilepsy population, and to determine whether OSA is associated with SUDEP risk profile.
Methods:
This study was approved by the Mayo Clinic Institutional Review Board. All participants provided informed consent for research participation in this prospective cross-sectional study. We enrolled consecutive adult patients with an epilepsy diagnosis admitted to the Mayo Clinic epilepsy monitoring unit. We excluded patients with psychogenic or other non-epileptic events. Patient subgroups included focal, generalized, or unclassified epilepsy syndromes. Probable OSA was identified using three measures: portable overnight oximetry (Palmsat 2500 Series, Nonin, Inc., Plymouth, Minnesota), Sleep Apnea—Sleep Disorder Questionnaire (SA-SDQ) and STOP-BAG.21, 22 From portable oximetry, the oxyhemoglobin desaturation index (ODI) was calculated as the number of automatically detected 4 percent or greater desaturation events, divided by hours of recording time, and ODI was also corrected for artifact and probable sleep time via concomitant wrist actigraphy derived estimated sleep times (Actiwatch, Phillips Respironics Motion Biosensors, Amsterdam, NL). We defined probable OSA as an adjusted ODI of ODI of ≥5/hour. The 12-item SA-SDQ questionnaire is a validated tool for sleep-disordered breathing by assessing OSA symptom severity. Frequency of OSA symptoms yield a score scale of 0–60, with OSA cutoffs of 36 for men and 32 for women in patients with epilepsy.21 STOP-BAG provides a predicted score for OSA risk based on the presence or absence of the following seven criteria: a history of disruptive Snoring, Tiredness, Observed breathing pauses, high blood Pressure; and elevated BMI (≥ 35 kg/m2), Age >50 years, and male sex (Gender). One point is scored for each present factor, and patients are classified into categories of low (0–2), intermediate (3–4) or high risk (5–7) for OSA. Patients with a STOP-BAG score of ≥ 3 were considered to have a diagnosis of probable OSA.22 For subject safety, all with positive screening scores for probable OSA on questionnaires or oximetry were advised to seek clinical sleep medicine consultation and confirmatory polysomnography, although polysomnography was not a formal part of this low resources pilot study. Demographic and epilepsy-related data concerning seizure type, frequency, duration of epilepsy, presence of absence of nocturnal seizures, presence or absence of ILAE defined treatment refractory epilepsy, current antiepileptic drug treatment at the time of admission, calculated antiepileptic drug load (sum of each patient subject’s antiepileptic drug daily dose, divided by that drug’s WHO defined daily dosage), other data necessary for calculation of rSUDEP-7 scores, and information concerning medical co-morbidites and other medications was abstracted from hospital admission notes.
Statistical Analyses.
Statistical calculations were performed using JMP Version 13 (SAS Inc., Cary, NC). Qualitative data were reported as relative frequencies. Quantitative data were reported as means, medians, standard deviation, and range. Chi square tests were used to compare categorical variables, while Wilcoxon rank-sum tests were utilized for continuous variables. Pearson’s correlation coefficient and multivariate regression analyses were then used to determine relationships between rSUDEP-7 scores and OSA measures. Significance level was set at an alpha of p < 0.05. Agreement between subjective (questionnaire) and objective (oximetric ODI) measures was assessed using the kappa statistic, with level of agreement specified according to standard definitions.23
Results:
49 consecutive epilepsy patient subjects were analyzed, 14 (28.6%) of which with treatment resistant epilepsy. The group had an average age of 37.2 ± 12.1 years, and average epilepsy duration of 16.7 ± 14.5 years. Patient demographics and clinical characteristics for the entire cohort are shown in Table 2. Nineteen (38%) patients had probable OSA (pOSA) by either oximetry, SA-SDQ, or STOP-BAG. 43 patients had analyzable oximetry (6 patients had technically inadequate oximetry studies), and of these, mean ODI was 4.65 ± 5.7 (range 0.1–22.8), with 15 (35%) having ODI≥5/hour (Figure 1). While all 19 subjects who screened positive were advised to pursue sleep medicine consultation and polysomnography, only 3/19 (15.8%) did so, and in these subjects OSA was confirmed, with a mean AHI of 6.3/hour. Comparisons between the subgroups of patients with (ODI≥5/hour) and without (ODI<5/hour) pOSA are also shown in Table 2. pOSA patients by oximetric ODI ≥5/hour had significantly higher SA-SDQ and STOP-BAG scores, were older, more likely to have focal epilepsy syndrome diagnosis and a longer duration of epilepsy than those who did not have pOSA by ODI (p all <0.05). In particular, patients with at least 30 years of epilepsy had significantly higher frequency of pOSA as identified by any measure compared with patients with epilepsy for less than 30 years (p <0.05). Agreement between STOP-BAG and ODI for probable OSA diagnosis was fair (k=0.39), while agreement between SA-SDQ and ODI was moderate (k=0.45). Agreement for pOSA diagnosis was best between SA-SDQ and STOP-BAG measures (k=0.66).
Table 2: Patient Demographics, Epilepsy and Sleep Variables.
The Table shows data for the overall cohort (n=49) and the subset of patients with analyzable oximetry data (n=43), comparing those with probable OSA having an ODI ≥ 5/hour, and those without probable OSA having ODI < 5/hour.
| All Patients (n=49) | Probable OSA (ODI ≥ 5, n= 15)a | No Probable OSA (ODI < 5, n = 28)b | p-value (a vs b) | |
|---|---|---|---|---|
| Age (years) | 37.2 ± 12.1 | 42.5 ± 13.5 | 33.6 ± 10.9 | 0.03 |
| Sex (M/F) | 22 / 27 | 8/7 | 11/17 | NS |
| Mean BMI (kg/m2) | 26.3 ± 6.1 | 29.5 ± 5.7 | 24.3 ± 5.2 | 0.005 |
| BMI>/= 30 kg/m2 | 13 (33.3%) | 8 (53.3%) | 7 (43.7%) | 0.002 |
| Mean rSUDEP-7 | 2.9 ± 1.4 | 3.4 ± 1.7 | 2.5 ± 1.1 | NS |
| Mean SUDEP-7 | 3.3 ± 1.7 | 3.7 ± 2.0 | 3.0 ± 1.5 | NS |
| Focal epilepsy n (%) | 36 (73%) | 14 (93%) | 17 (60%) | 0.01 |
| Primary Generalized epilepsy n (%) | 11 (22%) | 1 (7%) | 9 (32) | 0.04 |
| Unspecified-type epilepsy n (%) | 2 (4%) | 0 (0%) | 0 (0%) | NS |
| Nocturnal seizures n (%) | 18 (37%) | 4 (27%) | 13 (46%) | NS |
| Refractory epilepsy n (%) | 32 (65%) | 9 (60%) | 18 64%) | NS |
| Duration of epilepsy (years) | 16.7 ± 14.5 | 27.5 ± 15.7 | 10.9 ± 8.5 | 0.0005 |
| Duration of epilepsy >15 years n (%) | 15 (31%) | 10 (67%) | 5 (18%) | 0.0002 |
| Seizure frequency (days per month) | 10.4 ± 14.8 | 10.7 ± 12.4 | 10.8 ± 17.2 | NS |
| Anti-epileptic drugs | 2.2 ± 0.9 | 2.1 ± 1.0 | 2.2 ± 0.8 | NS |
| Anti-epileptic drug load | 2.6 ± 1.6 | 2.3 ± 1.9 | 2.6 ± 1.5 | NS |
| OSA Dx by Any Measure n (%) | 19 (39%) | NA | NA | |
| OSA Dx by Oximetry* | 15 (35%) | NA | NA | |
| ODI* | 4.7 ± 5.8 | 11.3 ± 5.1 | 1.1 ± 1.0 | <0.0001 |
| OSA Dx by SA-SDQ n (%) | 10 (20%) | 6 (40%) | 2 (7%) | 0.005 |
| SA-SDQ Score (range) | 22.2 ± 8.2 (13–49) | 27.6 ± 8.8 (17–49) | 19.0 ± 6.2 (13–37) | <0.0001 |
| OSA Dx by STOP-BAG | 6 (12%) | 8 (40%) | 2 (7%) | 0.009 |
| STOP-BAG Score (range) | 1.2 ± 1.1 (0–5) | 1.9 ± 0.9 (1–4) | 0.8 ± 0.9 (0–4) | <0.0001 |
| ESS | 7.6 ± 3.5 | 7.9 ±3.6 | 7.4 ± 3.6 | NS |
| Psychiatric Hx n (%) | 16 (33%) | 5 (33%) | 9 (32%) | NS |
| Hypertension/Hyperlipidemia n, (%) | 6 (12%) | 1 (7%) | 5 (18%) | NS |
| Migraine Hx n (%) | 11 (22%) | 4 (27%) | 6 (21%) | NS |
| TBI Hx n (%) | 7 (14%) | 1 (7%) | 4 (14%) | NS |
| Structural brain lesion n (%) | 6 (12%) | 1 (7%) | 3 (11%) | NS |
43 subjects had analyzable oximetry data
rSUDEP-7=Revised Sudden unexpected death in epilepsy score; BMI=body mass index; OSA=obstructive sleep apnea; Dx=diagnosis; Hx=History; TBI=Traumatic Brain Injury; ESS=Epworth sleepiness scale; ODI=oxygen desaturation index, SA-SDQ=sleep apnea-sleep disorders questionnaire
Figure 1. Two portable overnight oximetry profiles.
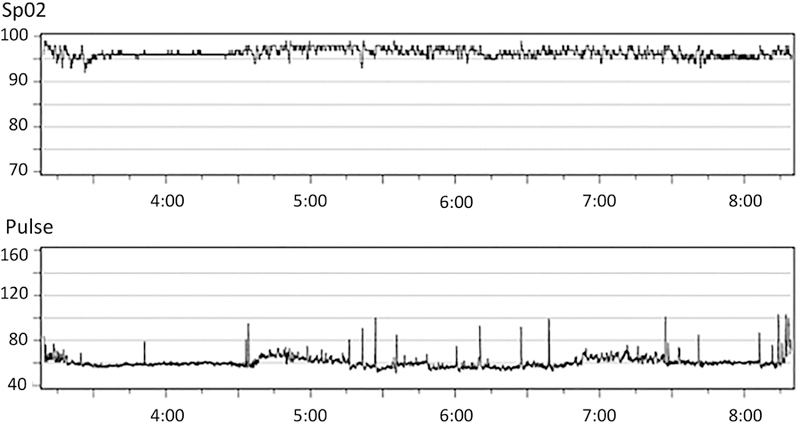
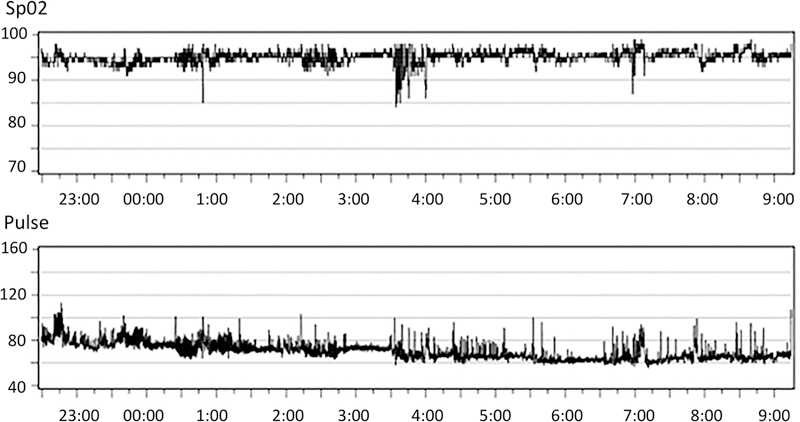
from (A) an epilepsy patient with a normal oxyhemoglobin desaturation index of < 1/ hour, and (B) an epilepsy patient with an ODI of 6/hour, showing several clustered oscillatory desaturations typical of mild obstructive sleep apnea.
Median rSUDEP-7 score was 3 (range 0–6), with mean of 2.9 ± 1.4. Higher rSUDEP-7 scores were associated with higher ODI values (Pearson’s r=0.32, p=0.036, Figure 2), and rSUDEP-7 scores >3 were also associated with pOSA diagnosis by ODI≥5/hour (univariate, p=0.036). Patients with a rSUDEP-7 score of ≥5 had higher adjusted ODI compared with scores of <5 (p=0.007), which remained significantly associated with probable OSA in multivariate analysis when adjusting for age, sex, calculated AED load, number of AEDs at time of study and BMI (p=0.03). Patients with a rSUDEP7 score ≥5 were more likely to have a diagnosis of OSA by all three measures combined (p=0.006), as well as ODI (p=0.01), STOP-BAG (p=0.04) and SA-SDQ (p=0.008), individually. There were also associations between rSUDEP-7 scores ≥5 and patients having a pOSA diagnosis by all 3 measures (oximetry, STOP-BAG, and SA-SDQ) (p=0.001). There were no associations between rSUDEP-7 score and age, sex, BMI, epilepsy type (partial versus generalized), nocturnal seizures, refractory epilepsy, or sleepiness.(Table 2) On the other hand, patients with a longer duration of epilepsy had higher rSUDEP-7 scores (p=0.008).
Figure 2. Relationship Between rSUDEP-7 Risk Profile and Adjusted ODI.
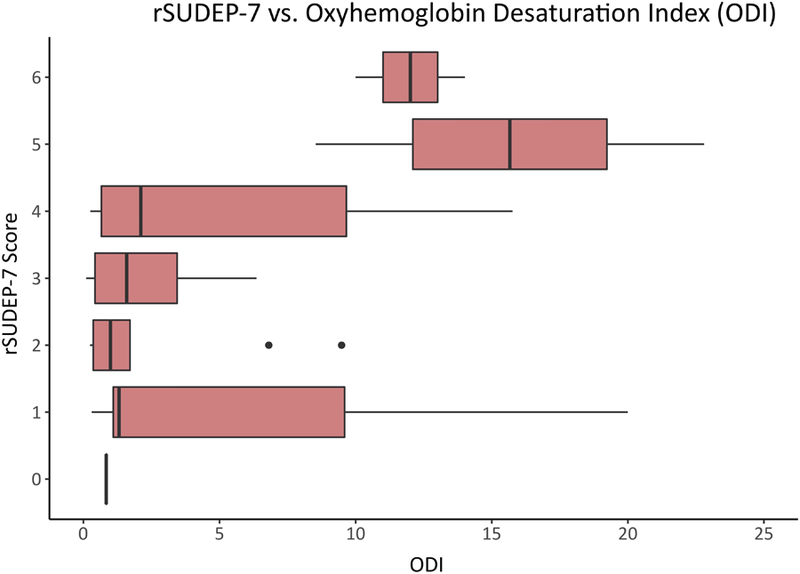
Shown is the association between SUDEP risk profile as indexed by scores on the rSUDEP-7, and the adjusted oxyhemoglobin desaturation index, where scores >5/hour are consistent with probable obstructive sleep apnea.
Discussion
Probable OSA was associated with rSUDEP-7 Inventory scores, and probable OSA measured objectively by overnight oximetry remained associated with rSUDEP-7 scores ≥5, when controlling for co-variates of age, sex, and BMI. Additionally, rSUDEP-7 scores ≥5 were associated with a probable OSA by the SA-SDQ and STOP-BAG questionnaire measures. Taken together, our results suggest that OSA is likely highly prevalent in inpatients with refractory epilepsy, and may be associated with an increased risk for sudden death in patients with epilepsy; thus identifying OSA as a possible modifiable risk factor for SUDEP.
Thirty-five percent of refractory epilepsy patients in our cohort had probable OSA by oximetry, while an even higher 39% of patients had probable OSA by either oximetry or one of the screening tools. The relatively high frequency of co-morbid probable OSA in our cohort is similar to prior studies of refractory epilepsy using polysomnography reported OSA frequencies of 22–30%.20;19;18Another study comparing polysomnographic variables between refractory and well-controlled epilepsy patients found that 20% of refractory epilepsy patients had an apnea-hypopnea index (AHI) of >5/hour, while no well-controlled epilepsy had OSA.24 A limitation of our pilot study was lack of gold-standard polysomnography for confirmation of OSA diagnosis, suggesting that the true frequency of OSA may have been even higher (since oximetric detection of mild OSA is variable, meaning our estimated frequency could be an underestimate).
Few studies have evaluated the effect of continuous positive airway pressure (CPAP) therapy on seizure frequency and outcomes. However, several retrospective studies have found that OSA-epilepsy patients treated with CPAP were more likely to have a significant reduction in seizure frequency, or remain seizure-free at follow-up, when compared with OSA-epilepsy patients not treated with CPAP.20, 25–27 Unsurprisingly, CPAP compliant patients appear to have more significant reduction in seizure frequency when compared with non-compliant patients.26 CPAP therapy also significantly reduced the frequency of interictal spikes compared with patients not using CPAP therapy, especially sleep-related spike frequency.28 A single randomized-controlled pilot study has also suggested efficacy for CPAP therapy in reducing seizure burden in epilepsy.29 Since previous evidence has suggested that treatment of co-morbid OSA reduces seizure frequency, and given our findings of a possible association between OSA and heightened SUDEP risk profile, co-morbid OSA should be identified early and treated in patients with epilepsy.
Given the nocturnal predominance of SUDEP cases, it is possible that OSA may potentiate the proposed mechanisms of SUDEP. The majority of witnessed SUDEP cases involve a terminal seizure followed by apnea preceding a fatal arrhythmia.11 Cardiac arrhythmias, one of the main proposed contributors to SUDEP, may be augmented by OSA. Sudden cardiac death, especially at night, has been associated with OSA in the general population without epilepsy, and may compound underlying autonomic dysfunction due to the comorbid seizure disorder.13 Several mechanisms have been proposed, including nocturnal hypoxemia that may result in ventricular ectopy and arrhythmia. Obstructive apneas and hypopneas may lead to hypercapnia-induced sympathetic surges and hypoxemia that increases myocardial oxygen demand, resulting in cardiac ischemia and potentially fatal dysrhythmias.13 Alternatively, the elevated sympathetic tone caused by chronic OSA may worsen ictal or post-ictal sympathetic surges, promoting tachyarrhythmias.11, 30 OSA has been shown to increase the QTc interval, increasing the risk of sudden cardiac death and SUDEP in vulnerable refractory epilepsy patients who may already have underlying prolonged QTc due to seizures or antiepileptic drug adverse effects.31;32 Finally, heart rate variability (HRV), a marker of autonomic function, was found to be decreased in patients with epilepsy (and also associated with higher SUDEP-7 scores), and if further impaired by OSA, could worsen poor preexisting underlying autonomic function in patients with epilepsy that favors development of tachydysrhythmias and sudden death. 33;34
Respiratory dysfunction, another hypothesized mechanism for SUDEP, may also be worsened by OSA. Both ictal and peri-ictal hypoxemia and hypercapnia secondary to central apnea have been reported in patients with partial and generalized epilepsy, promoted especially by prolonged focal seizures of temporal lobe origin and by generalized tonic-clonic seizures.35;36;37 Seizure spread to the amygdala appears to be important in the pathogenesis of ictal apnea.36 Serotonin (5-HT) receptors play an important role in ventilatory response to hypercapnia, and 5-HT receptor dysfunction has been hypothesized to play a role in the pathogenesis of SUDEP.38;39 OSA patients have been shown to have chronic hypercapnia with a decreased ventilatory response to higher carbon dioxide levels, particularly at night.40 As such, the combination of dysfunctional 5-HT neurons and blunted respiratory response to hypercapnia caused by chronic OSA may further reduce respiratory drive following a post-ictal apnea, increasing likelihood of persistent hypoxia leading to fatal arrhythmia and death.
Finally, OSA may contribute to SUDEP risk by increasing sleep fragmentation, and resultant sleep deprivation, lowering the seizure threshold and increasing the risk for refractory seizures, status epilepticus, and seizure related deaths.41 Taken together, these findings highlight the importance of early identification and treatment of sleep apnea, not only to decrease the augmentation of autonomic and respiratory dysfunction underlying the pathogenesis of SUDEP, but also to reduce sleep fragmentation and deprivation, which in turn could directly reduce the risk of SUDEP by improving seizure control.25; 28; 26
Our study has several limitations, including its small sample size drawn from a single tertiary referral center, making selection and referral biases possible, and its focus on refractory monitored epilepsy inpatients, potentially limiting generalizability to a broader population of epilepsy patients. In addition, we utilized the rSUDEP-7 risk profiling score as a surrogate for SUDEP risk, rather than the outcome of SUDEP itself, and while the SUDEP-7 has not been directly validated in prospective studies, it was derived from one of the largest and best prospective studies of SUDEP risk factors, and has been previously used to assess risk status for association with candidate SUDEP biomarkers of heart rate variability and cerebral hypoxia.5–6,9 Currently, well validated SUDEP risk profiling tools for use in living epilepsy patients are lacking, and this research gap should be addressed by further large scale population based studies. Additional limitations of our study were that we relied on retrospectively estimated seizure frequency counts provided by patient report and ad hoc availability of seizure diaries, rather than prospectively recorded seizure frequency by patient seizure diaries and objective measures to estimate seizure burden, potentially leading to misestimate of seizure counts. Future prospective studies should assess seizure frequency to correlate with SUDEP risk. Additionally, there were a relatively small number of our patient subjects had generalized seizures within the previous year, potentially impacting rSUDEP-7 scores and future SUDEP risk. However, our study population drawn from the epilepsy monitoring unit is likely representative of refractory epilepsy patients who are at highest risk for SUDEP, with a relatively large number (11, 28%) of the sample having treatment refractory epilepsy. Measurement of neck circumference was not available on all patients, so we utilized the STOP-BAG questionnaire rather than STOP-BANG. Since the association between probable OSA and rSUDEP-7 was significant even in the absence of consideration of neck circumference or a greater number of patients having more frequent generalized seizures, it may have been that utilization of neck circumference measures and STOP-BANG would have resulted in an even stronger association. However,, recent data suggests that the addition of neck circumference to the model may not have differed greatly, since in one recent study STOP-BANG and STOP-BAG did not differ significantly in the sensitivity and specificity for OSA diagnosis, so we believe the STOP-BAG model is likely sufficient for OSA diagnosis.42 Additionally, given that this was a limited resource pilot study, we were unable to definitively diagnose OSA with polysomnography. These findings need to be confirmed by a larger more definitive study in the EMU with polysomnography to better determine the true prevalence of OSA, the magnitude of association between OSA and SUDEP risk, and to explore possible mechanisms for SUDEP in at-risk refractory epilepsy patients including interictal, periictal, and ictal autonomic and respiratory alterations. We performed a post-hoc sample size calculation to determine the number of subjects with epilepsy necessary to determine a similar frequency of OSA as determined by this pilot study utilizing instead the gold standard measure for OSA of polysomnography, and including community controls to prospectively ascertain additional markers for SUDEP risk status. Assuming a comparable frequency of OSA of 34.8% found in this pilot study utilizing oximetry measures, we would need approximately 25 epilepsy patients and 25 controls to detect a significant group difference in OSA assuming community prevalent control OSA rates near 2–4% with 80% power at an alpha level of 0.05. Assuming more conservative frequencies of OSA in epilepsy ranging from 25–34%, or a higher frequency of OSA in community controls between 5–12%, this would increase the sample size necessary to show significant group differences in OSA to between 27–80 subjects per group.
Conclusion
Our pilot study reaffirms the high frequency of OSA in patients with epilepsy, finding that patients with probable OSA were older, heavier, with more frequent focal epilepsy syndrome and a longer duration of epilepsy. Our data also demonstrate a possible association between probable OSA and higher SUDEP risk profile. Future larger confirmatory studies with combined video-EEG-PSG for more definitive identification of OSA and delineating its relationship to interictal and perictal hypoxia, hypercapnia, and autonomic function are needed. Given the potential association between OSA, seizures, and SUDEP, patients with epilepsy should be screened and treated for OSA to reduce its impact on cardiorespiratory function and improve sleep consolidation.
Key Points:
Obstructive sleep apnea is common in monitored epilepsy inpatients.
Epilepsy patients with probable OSA were older, heavier, and more frequently had focal seizures and a longer duration of epilepsy.
Higher revised SUDEP-7 scores are associated with probable OSA.
Additional confirmatory studies with polysomnography are needed to determine whether OSA is associated with SUDEP risk.
ACKNOWLEDGEMENT:
The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 1 UL1 RR024150–01. VKS and EKS receive support from NIH HL-65176. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We are additionally grateful for secretarial support in manuscript preparation and submission from Ms. Lea Dacy, Mayo Clinic Department of Neurology.
Disclosures:
Study Funding: Supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 1 UL1 RR024150–01. CAAC and VKS are supported by NIH HL65176 and NIH HL134885. CAAC is supported by the American Heart Association (Award number 17POST33400211).
VK Somers reports that he receives research support from NIH HL-65176. He is responsible for critical review of manuscript for content.
EK St. Louis reports that he receives research support from the Mayo Clinic Center for Translational Science Activities (CCaTS), supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 1 UL1 RR024150–01, and from NIH HL-65176.. He is responsible for study concept/design, data acquisition, analysis, and interpretation, and authorship of manuscript.
Footnotes
Publisher's Disclaimer: This manuscript describes original work of the aforementioned authors. It has not been previously published and is not being simultaneously considered by another journal. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
No off-label medication use.
Disclosures:
AR McCarter reports no disclosures. She is responsible for data analysis and interpretation of data, and authorship of manuscript.
PC Timm reports no disclosures. He is responsible for data collection, analysis, and critical review of manuscript for content.
PW Shepard reports no disclosures. He is responsible for data collection and critical review of the manuscript for content.
DJ Sandness reports no disclosures. He is responsible for data collection and critical review of manuscript for content.
T Luu reports no disclosures. She is responsible for analysis of data and critical review of manuscript for content.
SJ McCarter reports no disclosures. He is responsible for data collection, analysis, and critical review of manuscript for content.
L Dueffert reports no disclosures. He is responsible for data collection and critical review of manuscript for content.
M Dresow reports no disclosures. He is responsible for critical review of manuscript for content.
J Feemster reports no disclosures. He is responsible for analysis of data analysis and critical review of manuscript for content.
GD Cascino reports no disclosures. He is responsible for critical review of manuscript for content.
EL So reports no disclosures. He is responsible for critical review of manuscript for content.
GA Worrell reports no disclosures. He is responsible for critical review of manuscript for content.
JW Britton reports no disclosures. He is responsible for critical review of manuscript for content.
A Sherif reports no disclosures. He is responsible for critical review of manuscript for content.
K Jaliparthy reports no disclosures. He is responsible for analysis of data and critical review of manuscript for content.
CAA Chahal reports no disclosures. He is responsible for critical review of manuscript for content.
References:
- 1.Ficker DM, So EL, Shen WK, et al. Population-based study of the incidence of sudden unexplained death in epilepsy. Neurology 1998;51:1270–1274. [DOI] [PubMed] [Google Scholar]
- 2.Nashef L, So EL, Ryvlin P, Tomson T. Unifying the definitions of sudden unexpected death in epilepsy. Epilepsia 2012;53:227–233. [DOI] [PubMed] [Google Scholar]
- 3.Shorvon S, Tomson T. Sudden unexpected death in epilepsy. Lancet 2011;378:2028–2038. [DOI] [PubMed] [Google Scholar]
- 4.Massey CA, Sowers LP, Dlouhy BJ, Richerson GB. Mechanisms of sudden unexpected death in epilepsy: the pathway to prevention. Nat Rev Neurol 2014;10:271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walczak TS, Leppik IE, D’Amelio M, et al. Incidence and risk factors in sudden unexpected death in epilepsy: a prospective cohort study. Neurology 2001;56:519–525. [DOI] [PubMed] [Google Scholar]
- 6.DeGiorgio CM, Miller P, Meymandi S, et al. RMSSD, a measure of vagus-mediated heart rate variability, is associated with risk factors for SUDEP: the SUDEP-7 Inventory. Epilepsy Behav 2010;19:78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novak JL, Miller PR, Markovic D, Meymandi SK, DeGiorgio CM. Risk Assessment for Sudden Death in Epilepsy: The SUDEP-7 Inventory. Front Neurol 2015;6:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.So EL. What is known about the mechanisms underlying SUDEP? Epilepsia 2008;49 Suppl 9:93–98. [DOI] [PubMed] [Google Scholar]
- 9.Moseley BD, Britton JW, Nelson C, Lee RW, So E. Periictal cerebral tissue hypoxemia: a potential marker of SUDEP risk. Epilepsia 2012;53:e208–211. [DOI] [PubMed] [Google Scholar]
- 10.Rajakulendran S, Nashef L. Postictal generalized EEG suppression and SUDEP: a review. J Clin Neurophysiol 2015;32:14–20. [DOI] [PubMed] [Google Scholar]
- 11.Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol 2013;12:966–977. [DOI] [PubMed] [Google Scholar]
- 12.Wandschneider B, Koepp M, Scott C, et al. Structural imaging biomarkers of sudden unexpected death in epilepsy. Brain 2015;138:2907–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gami AS, Olson EJ, Shen WK, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol 2013;62:610–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005;352:1206–1214. [DOI] [PubMed] [Google Scholar]
- 15.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep 2008;31:1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 16.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 17.Manni R, Terzaghi M, Arbasino C, Sartori I, Galimberti CA, Tartara A. Obstructive sleep apnea in a clinical series of adult epilepsy patients: frequency and features of the comorbidity. Epilepsia 2003;44:836–840. [DOI] [PubMed] [Google Scholar]
- 18.Phillips MC, Costello CA, White EJ, et al. Routine polysomnography in an epilepsy monitoring unit. Epilepsy Res 2013;105:401–404. [DOI] [PubMed] [Google Scholar]
- 19.Malow BA, Levy K, Maturen K, Bowes R. Obstructive sleep apnea is common in medically refractory epilepsy patients. Neurology 2000;55:1002–1007. [DOI] [PubMed] [Google Scholar]
- 20.Foldvary-Schaefer N, Andrews ND, Pornsriniyom D, Moul DE, Sun Z, Bena J. Sleep apnea and epilepsy: who’s at risk? Epilepsy Behav 2012;25:363–367. [DOI] [PubMed] [Google Scholar]
- 21.Weatherwax KJ, Lin X, Marzec ML, Malow BA. Obstructive sleep apnea in epilepsy patients: the Sleep Apnea scale of the Sleep Disorders Questionnaire (SA-SDQ) is a useful screening instrument for obstructive sleep apnea in a disease-specific population. Sleep Med 2003;4:517–521. [DOI] [PubMed] [Google Scholar]
- 22.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008;108:812–821. [DOI] [PubMed] [Google Scholar]
- 23.Gisev N, Bell JS, Chen TF. Interrater agreement and interrater reliability: key concepts, approaches, and applications. Res Social Adm Pharm 2013;9:330–338. [DOI] [PubMed] [Google Scholar]
- 24.Zanzmera P, Shukla G, Gupta A, et al. Markedly disturbed sleep in medically refractory compared to controlled epilepsy - a clinical and polysomnography study. Seizure 2012;21:487–490. [DOI] [PubMed] [Google Scholar]
- 25.Pornsriniyom D, Kim H, Bena J, Andrews ND, Moul D, Foldvary-Schaefer N. Effect of positive airway pressure therapy on seizure control in patients with epilepsy and obstructive sleep apnea. Epilepsy Behav 2014;37:270–275. [DOI] [PubMed] [Google Scholar]
- 26.Vendrame M, Auerbach S, Loddenkemper T, Kothare S, Montouris G. Effect of continuous positive airway pressure treatment on seizure control in patients with obstructive sleep apnea and epilepsy. Epilepsia 2011;52:e168–171. [DOI] [PubMed] [Google Scholar]
- 27.St Louis EK. Diagnosing and Treating Co-morbid Sleep Apnea in Neurological Disorders, Part II. Pract Neurol (Fort Wash Pa) 2010;9:26–31. [PMC free article] [PubMed] [Google Scholar]
- 28.Pornsriniyom D, Shinlapawittayatorn K, Fong J, Andrews ND, Foldvary-Schaefer N. Continuous positive airway pressure therapy for obstructive sleep apnea reduces interictal epileptiform discharges in adults with epilepsy. Epilepsy Behav 2014;37:171–174. [DOI] [PubMed] [Google Scholar]
- 29.Malow BA, Foldvary-Schaefer N, Vaughn BV, et al. Treating obstructive sleep apnea in adults with epilepsy: a randomized pilot trial. Neurology 2008;71:572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995;96:1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamberts RJ, Blom MT, Novy J, et al. Increased prevalence of ECG markers for sudden cardiac arrest in refractory epilepsy. J Neurol Neurosurg Psychiatry 2015;86:309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura T, Chin K, Hosokawa R, et al. Corrected QT dispersion and cardiac sympathetic function in patients with obstructive sleep apnea-hypopnea syndrome. Chest 2004;125:2107–2114. [DOI] [PubMed] [Google Scholar]
- 33.Lotufo PA, Valiengo L, Bensenor IM, Brunoni AR. A systematic review and meta-analysis of heart rate variability in epilepsy and antiepileptic drugs. Epilepsia 2012;53:272–282. [DOI] [PubMed] [Google Scholar]
- 34.Roche F, Xuong AN, Court-Fortune I, et al. Relationship among the severity of sleep apnea syndrome, cardiac arrhythmias, and autonomic imbalance. Pacing Clin Electrophysiol 2003;26:669–677. [DOI] [PubMed] [Google Scholar]
- 35.Bateman LM, Li CS, Seyal M. Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity and risk factors. Brain 2008;131:3239–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dlouhy BJ, Gehlbach BK, Kreple CJ, et al. Breathing Inhibited When Seizures Spread to the Amygdala and upon Amygdala Stimulation. J Neurosci 2015;35:10281–10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seyal M, Bateman LM. Ictal apnea linked to contralateral spread of temporal lobe seizures: Intracranial EEG recordings in refractory temporal lobe epilepsy. Epilepsia 2009;50:2557–2562. [DOI] [PubMed] [Google Scholar]
- 38.Li A, Nattie E. Serotonin transporter knockout mice have a reduced ventilatory response to hypercapnia (predominantly in males) but not to hypoxia. J Physiol 2008;586:2321–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richerson GB, Buchanan GF. The serotonin axis: Shared mechanisms in seizures, depression, and SUDEP. Epilepsia 2011;52 Suppl 1:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan H, Pinto SJ, Huang J, et al. Ventilatory responses to hypercapnia during wakefulness and sleep in obese adolescents with and without obstructive sleep apnea syndrome. Sleep 2012;35:1257–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malow BA. Sleep deprivation and epilepsy. Epilepsy Curr 2004;4:193–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katzan IL, Thompson NR, Uchino K, Foldvary-Schaefer N. A screening tool for obstructive sleep apnea in cerebrovascular patients. Sleep Med 2016;21:70–76. [DOI] [PubMed] [Google Scholar]


