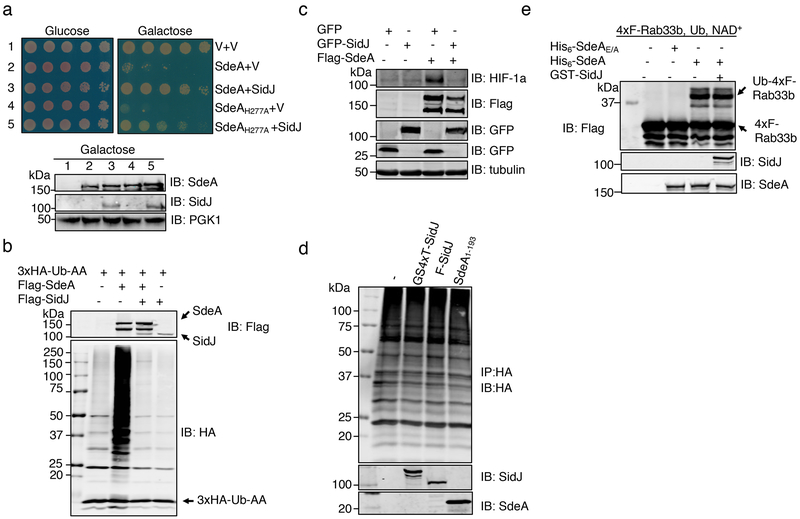
Fig. 1. SidJ antagonizes the effects of SdeA in eukaryotic cells.
a. SidJ suppresses the yeast toxicity of SdeAH277A. Diluted cells from yeast strains inducible expressing SdeA or SdeAH277A that harbor the vector or a SidJ construct were spotted onto the indicated media and grew for 2 d (top). The expression of relevant proteins was probed by immunoblotting (bottom). The 3-phosphoglyceric phosphokinase-1 (PGK1) was probed as a loading control. V, vector.
b. SidJ abrogates SdeA-mediated ubiquitination in mammalian cells. Lysates of HEK293T cells expressing the indicated proteins were detected by immunoblotting with an HA-specific antibody to detect 3xHA-Ub-AA and proteins modified by 3xHA-Ub-AA. The expression of Flag-SdeA and Flag-SidJ was also probed.
c. SidJ rescues HIF-1α degradation blocked by SdeA. Lysates of HEK293T cells expressing the indicated proteins were resolved by SDS-PAGE and probed with antibodies specific for the epitope tags or relevant proteins.
d. SidJ from E. coli or HEK293T cells cannot deubiquitinate proteins modified by SdeA. Proteins modified by 3xHA-Ub-AA obtained by immunoprecipitation were treated with GST-SidJ from E. coli, Flag-SidJ from HEK293T or SdeA1-193. Note that none of these proteins caused a reduction in the ubiquitination signals.
e. GST-SidJ does not inhibit SdeA-induced ubiquitination in vitro. SidJ was co-incubated with SdeA for 2 h at 37°C and SdeA activity was assayed. A Flag-specific antibody was used to detect modified and unmodified 4xFlag-Rab33b, judging by a shift in its molecular weight. SdeA and SidJ were probed with specific antibodies. SdeAE/A is a SdeA mutant defective in the mART activity carrying E860A and E862A mutations. The experiment in each panel was performed independently for at least 3 times with similar results.