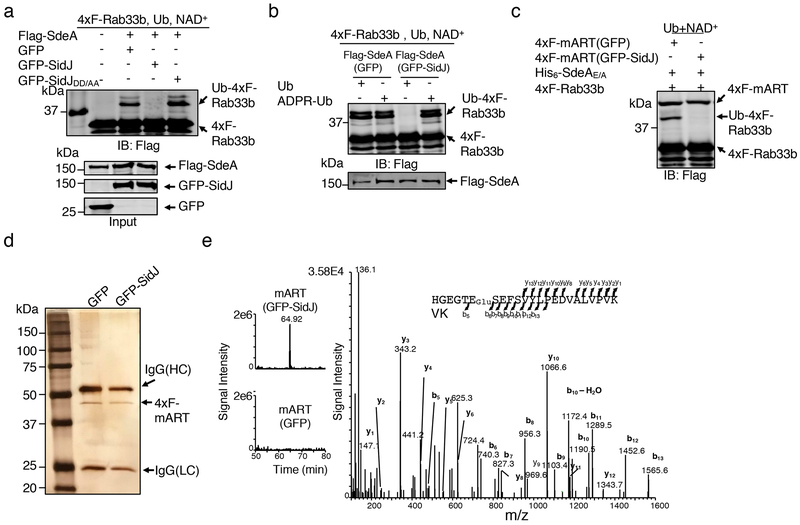
Fig. 2. SidJ post translationally modifies SdeA in mammalian cells and inhibits its activity to catalyze the production of ADP-ribosylated ubiquitin.
a. Flag-SdeA coexpressed with SidJ fails to modify Rab33b. Flag-SdeA from HEK293T cells coexpressing relevant proteins was used to ubiquitinate 4xFlag-Rab33b. Ub-Rab33b was detected as described in Fig. 1. SidJDD/AA is a SidJ mutant defective in suppressing the yeast toxicity of SdeA that carries D542A and D545A mutations.
b. Flag-SdeA coexpressed with SidJ retains the ability to ubiquitinate Rab33b with ADPR-Ub. ADPR-Ub or ubiquitin was incubated with Flag-SdeA purified from HEK293T cells coexpressing GFP or GFP-SidJ. NAD+ was included in reactions receiving ubiquitin. Rab33b modification was detected with a Flag-specific antibody.
c. SidJ attacks the mART activity of SdeA. 4xFlag-mART (SdeA563-910) purified from HEK293T cells coexpressing GFP or GFP-SidJ was incubated with 4xFlag-Rab33b, ubiquitin, NAD+ and His6-SdeAE/A for 2 h at 37°C before ubiquitination detection.
d-e. SidJ induces a 129.04 Dalton post-translational modification on E860 of SdeA. Mass spectrometric analysis of 4xFlag-mART* (d) identified a posttranslational modification in the fragment -H855GEGTESEFSVYLPEDVALVPVK877− (e, left panel). Tandem mass (MS/MS) spectrum shows the fragmentation profile of the modified peptide -H855GEGTEGluSEFSVYLPEDVALVPVK877−, including ions b5 and b6 that confirm the modification site at E860 (e, right panel). The experiment in each panel was repeated three times with similar results.