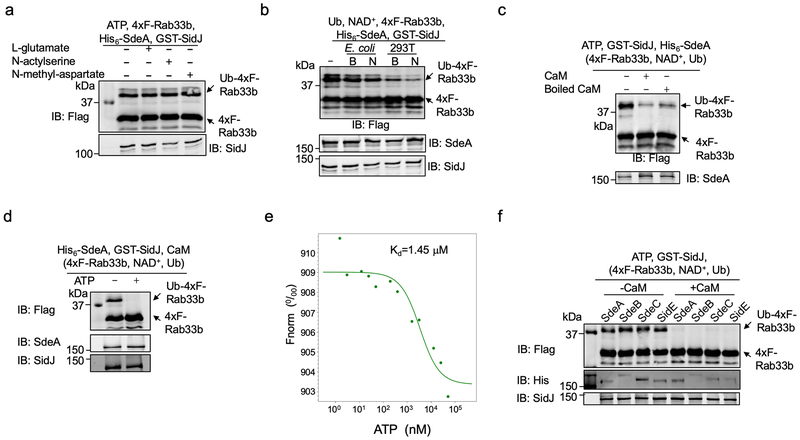
Extended Data Fig. 2. The effects of cell lysates, ATP, heat treatment on CaM on the activity of SidJ and its inhibition of the activity of all members of the SidE family.
a. Inhibition of SdeA activity does not occur in in vitro reactions containing L-glutamate or each of its two structural isomers. L-glutamate, N-acetylserine or N-methyl-aspartate was incubated with SdeA, SidJ and ATP for 2 h before assaying for the activity of SdeA.
b. A molecule(s) from mammalian cells is required for SidJ to inhibit SdeA. Lysates from E. coli or HEK293T cells were added to reactions containing SdeA and SidJ for 2 h before measuring the activity of SdeA.
c. Heat treatment does not completely abolish CaM activity. CaM or CaM treated by heating at 100°C for 5 min was included in reactions that allow glutamylation of SdeA for 2 h. A cocktail containing 4xF-Rab33b, NAD+ and ubiquitin was added to each reaction. Samples were resolved by SDS-PAGE and detected for Rab33b ubiquitination after another 2 h incubation at 37°C.
d. The activity of SidJ requires ATP. His6-SdeA was incubated with GST-SidJ, L-glutamate and CaM in reactions with or without 1 mM ATP for 2 h, 4xF-Rab33b, NAD+ and ubiquitin were added to each reaction. After another 2 h incubation, the activity of SdeA was evaluated by the production of ubiquitinated Ra33b. Protein components in the reactions were detected by immunoblotting with specific antibodies.
e. The binding of ATP by SidJ. Binding of ATP by purified SidJ was evaluated using Microscale thermophoresis in which the concentration of SidJ was kept constant. The dissociation constant (Kd) was determined by the NanoTemper Analysis 2.2.4 software.
f. SidJ inhibits the activity of members of the SidE family. Recombinant protein of each of SidE family protein was incubated with ATP, L-glutamate and GST-SidJ in the presence or absence of CaM for 2 h, a cocktail containing 4xF-Rab33b, NAD+ and ubiquitin was added to the reactions. After additional 2 h incubation, modification of Rab33b was detected by immunoblotting with a Flag-specific antibody. The formation of Ub-4xF-Rab33b is indicated by a shift in molecular weight. In each panel, data shown were one representative from at least three independent experiments that had similar results.