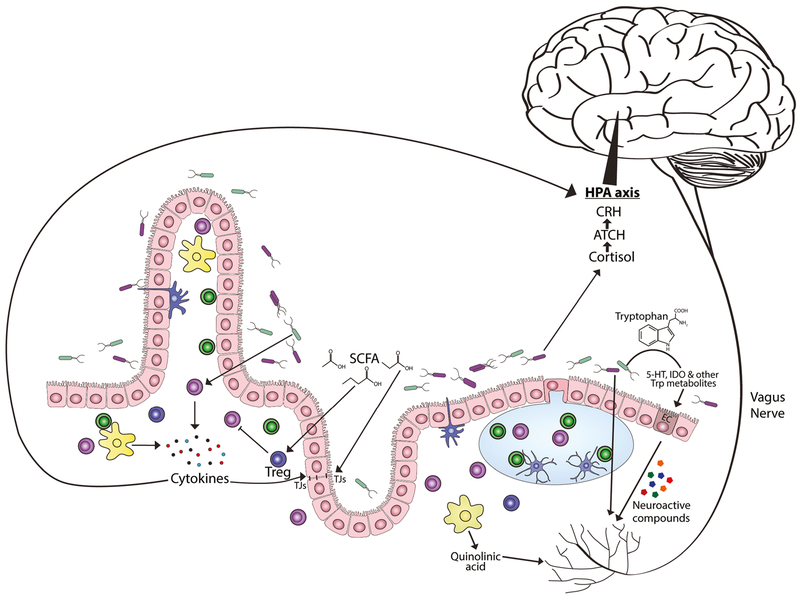
Fig. 1.
Mechanisms of the microbiota-gut-brain axis. Possible mechanisms of the MGB-axis are being actively investigated and include neuroimmune pathways, neural communication through the vagus nerve, influence of metabolites produced by the microbiota, microbial-derived neurotransmitters, and the significant influence the microbiota have on tryptophan, kynurenine, and serotonin metabolism. Short-chain fatty acids (SCFA) can promote peripheral T regulatory (Treg) cell expansion as well as influence tight junction (TJ) proteins and intestinal barrier function. Microbiota regulate tryptophan (Trp) metabolites by degrading Trp to indole-derivatives or through kynurenine and serotonin pathways, such as increasing expression of tryptophan hydroxylase (Tph)1 in enterochromaffin (EC) cells. Dysbiosis can promote activation of immune cells, including macrophages that produce quinolinic acid (QA) through an alternative kynurenine pathway, a known excitotoxic N-methyl-D-aspartate (NMDA) receptor agonist. Activated immune cells also produce proinflammatory cytokines which can further disrupt microflora and impact intestinal barrier function. Neural communication can also occur through the vagus nerve via signaling from hormones and neurotransmitters release by gut endocrine cells and immune cells. Breech of the intestinal barrier would also allow direct pattern recognition sensing due to Toll-like receptor expression on afferent fibers