Abstract
Humans are changing the physical properties of Earth. In marine systems, elevated carbon dioxide concentrations are driving notable shifts in temperature and seawater chemistry. Here, we consider consequences of such perturbations for organism biomechanics and linkages amongst species within communities. In particular, we examine case examples of altered morphologies and material properties, disrupted consumer–prey behaviours, and the potential for modulated positive (i.e. facilitative) interactions amongst taxa, as incurred through increasing ocean acidity and rising temperatures. We focus on intertidal rocky shores of temperate seas as model systems, acknowledging the longstanding role of these communities in deciphering ecological principles. Our survey illustrates the broad capacity for biomechanical and behavioural shifts in organisms to influence the ecology of a transforming world.
Keywords: Behavioural responses, functional ecology, global environmental change, global warming, ocean acidification, species interactions
Lay Summary: Brief overview of biomechanical and behavioural consequences of ocean acidification and warming for marine organisms and communities inhabiting rocky temperate shores.
Introduction
Humans are now the greatest agent of ecological change globally. Effects span those due to habitat loss, increased exchange of taxa across space, pollution, altered nutrient cycling, overfishing, reductions in top predators, as well as fundamental shifts in the properties of Earth’s atmosphere and oceans associated with emissions of carbon dioxide (Vitousek et al., 1997; Jackson et al., 2001; Estes et al., 2011). In marine systems, the latter perturbations drive especially important modifications to temperature and seawater chemistry (Feely et al., 2004; Harley et al., 2006; Doney et al., 2009). These physical changes affect biomechanical and behavioural processes relevant to organisms, including ones that alter the ecology of sea creatures and the communities in which they live.
Considerable work documents how marine life is affected by ocean warming and ocean acidification (OA; i.e. human-induced decreases in seawater pH and carbonate ion concentrations accompanied by elevated aqueous CO2; Caldeira and Wickett, 2003). Much of this research focuses on responses of individual taxa to these changes, centering on demographically relevant parameters like growth, survival and reproduction. Biomechanical consequences have received less attention. Similarly, implications for interactions amongst species remain underexplored, especially in OA research where studies primarily address effects on single species in isolation (Kroeker et al., 2010, 2013b). Although growing numbers of experiments examine the capacity of OA to alter behaviour (a core component of interactions amongst animals; see, e.g. Dixson et al., 2010; Watson et al., 2013; Jellison et al., 2016; Jellison and Gaylord, 2019), substantial knowledge gaps persist. Studies addressing effects of rising temperature incorporate a deeper functional and ecological perspective, but also highlight mostly shifts in demography and distribution (Poloczanska et al., 2013). Examinations of how environmental change may alter biomechanical and behavioural attributes of organisms, and accompanying relationships amongst taxa remain limited.
Effects of global change, however, are most apparent when the form, function and performance of organisms are evaluated in the context of the networks of species connections present within communities. Even when implications of altered temperature or chemistry apply most obviously to individual species, linkages amongst taxa pertain (Gaylord et al., 2015). Mechanism at the organismal scale influences species interactions at the ecological scale, with broader implications for natural systems. Indeed, marked ecological transitions associated with climate change are often mediated through shifts in organism-level traits and species interactions. Such ecological shifts do not necessarily arise just because a particular species exhibits susceptibility, but because the interplay between that species and others becomes disrupted, sparking an amplified response. Non-linearities intrinsic to such perturbations create thresholds, tipping points and even ecological phase shifts, where a system flips to another state and manner of functioning (Scheffer et al., 2001). Examples include warming-induced range expansions in sea urchins that induce widespread kelp deforestation (Ling, 2008; Ling et al., 2015), coral bleaching driven by high ocean temperature that causes ecological phase shifts in reef fish assemblages (Bellwood et al., 2006), and trophic cascades in rocky bottom communities deriving from climate-associated disease outbreaks in echinoderms (Schultz et al., 2016; Harvell et al., 2019).
Here, we address additional examples where biomechanical and behavioural consequences of environmental change link responses of organisms at the individual level to broader ecological concerns. We focus on temperate rocky intertidal zones, where physical–biological processes have been studied for decades, and where important ecological concepts have been elaborated. For the purposes of our overview, we discuss the current state of knowledge regarding climate stressors in such habitats, with more in-depth consideration of examples from our own studies. We focus especially on benthic stages of organisms, while also incorporating insights from larval studies given the importance of these dispersing stages for population structure and dynamics (e.g. Gaylord and Gaines, 2000; Gaylord et al., 2006; O’Connor et al., 2007; Byrne and Przeslawski, 2013; Nickoks et al., 2015). Our framework for discussion accounts explicitly for the capacity of biomechanical and behavioural traits of organisms to translate impacts through species interactions and drive broader changes in community and ecosystem properties (Fig. 1).
Figure 1.
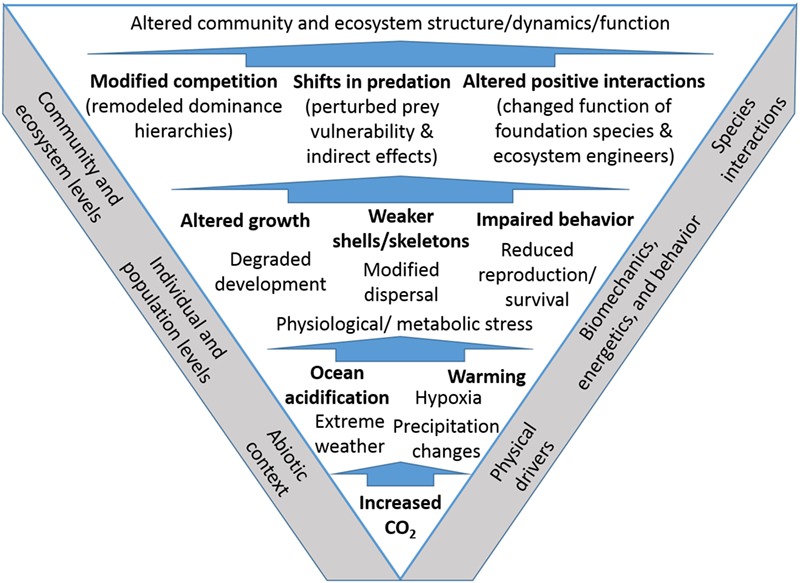
Conceptual diagram highlighting how effects of elevated emissions of carbon dioxide (CO2) can cascade upwards from lower levels of biological organization, through interactions amongst species, to ultimately influence community and ecosystem-scale properties. Although common consequences of elevated CO2 are depicted, they are not exhaustive; subjects shown in bold are explored in this review.
Environmental change and rocky shores
Intertidal habitats on open coasts experience dramatic swings in temperature and desiccation state as the tides rise and fall, as well as large forces imposed by rapid water velocities (see Denny and Gaylord, 2010; or Denny, 2016 for a biomechanical perspective). These challenges are exacerbated as climatic warming and shifts in wind patterns produce stronger thermal forcing and bigger ocean waves in some areas (Young et al., 2011; Reguero et al., 2019). OA operates as an additional, emerging threat (Orr et al., 2005; Kroeker et al., 2013a; Gaylord et al., 2015; Nagelkerken and Connell, 2015).
The physical stresses of rocky intertidal habitats also provide a backdrop for processes of predation, competition and facilitation that further define the structure and dynamics of these communities, and which have been well studied (see also Gaylord et al., 2015). Indeed, the importance of predation and herbivory (Paine, 1969; Lubchenco, 1978; Power et al., 1996), roles of competition and disturbance (Connell, 1961; Dayton, 1971; Sousa, 1979), the capacity for recruitment and environmental stress to modulate effects of predation and competition (Menge and Sutherland, 1987), all have been researched intensely in rocky intertidal environments. Understanding of facilitation (Stachowicz, 2001; Bruno et al., 2003) and habitat provisioning by foundation species (Dayton, 1972), or habitat modification by ecosystem engineers (Jones et al., 1994), has likewise benefitted from research conducted in shoreline communities (Lafferty and Suchanek, 2016; Jurgens and Gaylord, 2018).
The close connection between physical attributes of intertidal environments, the functional traits of organisms (including biomechanical and behavioural ones), and key ecological processes is also complicated by the geographic variation that exists in thermal and chemical stressors active within these areas. For instance, latitudinal gradients in solar input combine with variation in the timing of low tides to create mosaics of temperature extremes in intertidal habitats along the US west coast (Helmuth et al., 2002, 2006). Analogous mosaics in OA-related conditions arise due to regional variation in prevailing winds and oceanographic responses to those winds (Feely et al., 2008, 2016; Chan et al., 2017). Such variation, and accompanying differences in species composition across space (Abbott and Hollenberg, 1976; Morris et al., 1980; Carlton, 2007; see also Sanford et al., 2019), creates strong impetus to understand how organisms interact within the community assemblages that characterize a given location within the broader coastal landscape.
Ocean perturbations and biomechanics of predation
Amongst the most obvious ecological consequences of altered temperature and seawater chemistry are those tied to animal feeding and consumption. Energetic, biomechanical and behavioural considerations apply, of which we begin with the first two. Temperature affects physiology, including metabolic rate, energy demands, and often the impetus for, and intensity with which, marine animals forage (Schmidt-Nielsen, 1997; Hoffmann and Todgham, 2010; Somero, 2010). From the perspective of prey, seawater chemistry—in particular properties tied to the carbonate system and saturation state of calcium carbonate—influences the ability of calcifying taxa to precipitate shells, spines and skeletons (Ries et al., 2009). Such structural elements are essential features of predator deterrence (Vermeij, 1982).
There are clear examples where ocean change may impinge on processes underlying predation, including those affecting key community members. Consider California mussels (Mytilus californianus), which reach high abundances and operate as a major space holder in mid-intertidal regions on rocky shores of the eastern Pacific Ocean. They are often viewed as a competitive dominant amongst larger macro-fauna (Paine, 1966). At the same time, the complex, 3D matrix of the beds formed by this mussel provides habitat and shelter for hundreds of other species (Suchanek, 1992; Lafferty and Suchanek, 2016). Thus, this taxon serves as an ecosystem engineer that facilitates the success of many other organisms, making factors that influence its susceptibility to predation highly relevant to the community.
Our research and that of others implicates OA and elevated water temperatures as agents by which the biomechanical vulnerability of California mussels to consumption may change. In laboratory experiments, larval mussels exposed to elevated-CO2 (or lower-pH) seawater precipitate smaller shells, as well as shells that are thinner and weaker. These effects are revealed through standard imaging techniques such as scanning electron microscopy and classic biomechanical tests of breaking strength (Figs 2 and 3; Gaylord et al., 2011). Moreover, larval mussels cultured under OA conditions also produce less internal tissue for their body size (Fig. 3). Analogous experiments by Frieder et al. (2014) recapitulate a number of these trends, as do field studies conducted by our group concerning newly settled larval recruits. Together these experiments indicate the potential for altered interactions of young mussels with predators in the face of ocean change. Because larval mussels retain their shells when they complete their pelagic larval phase and settle into the benthos, degraded shell integrity or reduced body mass may impose a suite of costs on small juveniles. For example, new settlers must survive attacks by crushing and drilling predators that inhabit the beds formed by adult mussels (Gosselin and Qian, 1997). Shells weakened by OA may be less effective at resisting the chelae of the many juvenile crabs, for instance, that live within the matrix of mussel beds. Similarly, thinner shells may require less time and effort for carnivorous, drilling gastropods to penetrate (e.g. Kroeker et al., 2014b), an effect that may be exacerbated in warmer conditions (Miller, 2013). Decreased tissue mass could encourage higher attack rates by consumers, by decreasing the energetic reward associated with a given mussel individual and thereby driving predators to consume more individuals per time (Sanford et al., 2014).
Figure 2.
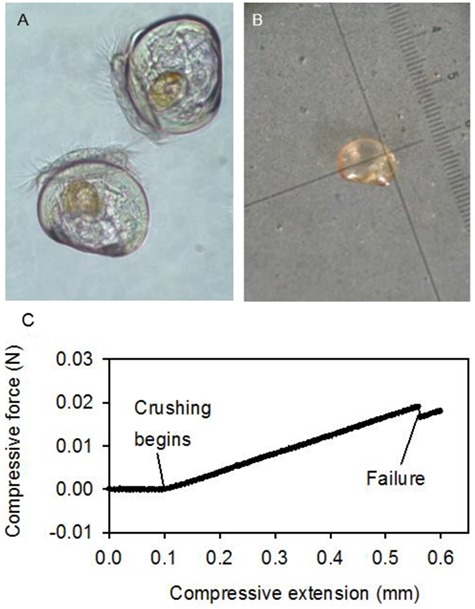
Biomechanical properties of the shells of larval mussels (Mytilus californianus) can be tested shortly before settlement into the benthos, as a function of their exposure to climate change drivers. (A) Veliger stage mussel larvae. The long axis of each of these individuals is ~120 μm. (B) Individual larvae can be mounted on microscope slides and the compressive force required to break their shells quantified, using a micro-force applicator affixed to a materials testing instrument. Smallest scale division equals 12.5 μm. (C) Example force trace during a representative test. Data and images redrawn after Gaylord et al. (2011).
Figure 3.
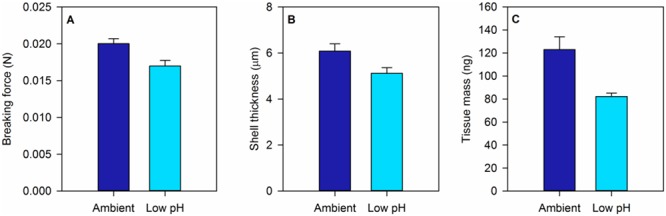
OA can cause late-stage mussel larvae (Mytilus californianus) to produce weaker and thinner shells, along with bodies of reduced tissue mass. Ambient seawater conditions were characterized by a pH of 7.9 (quantified on the total scale), and low-pH conditions by a pH of 7.6. Error bars indicate SEM. Further experimental details and full dataset can be found in Gaylord et al. (2011).
Negative effects of OA on shell properties may also apply to older and larger stages of mussels. Reciprocal transplants with mid-sized juvenile M. californianus across multiple sites along the west coast of North America show elevated susceptibility to predation by drilling whelks in regions of greater OA stress, if high levels of food are unavailable, or in especially warm locations (Kroeker et al., 2016). Moreover, shell thickness of large, adult California mussels has decreased over time, such that shells from the present day are significantly thinner than ones collected during the 1970s and from Native American middens dated to 1000–2400 years ago (Pfister et al., 2016). Additional data indicate accompanying changes in mussel shell mineralogy over the past 15 years (McCoy et al., 2018). It would be valuable to know whether frequencies of drill holes from predatory gastropods have risen over time in accordance with such thinning and changes in mineralogical properties.
Interspecific variation in biomechanical responses
While mussels appear to experience increased susceptibility to predation under environmental change, not all shoreline taxa will respond the same way. Paired work on two common gastropods found in intertidal habitats along the US west coast highlights disparate responses amongst taxa. In a long-term exposure of the herbivorous gastropod, Tegula funebralis (the black turban snail), and a predatory counterpart, Nucella ostrina (the striped dogwhelk), to OA, Barclay et al. (2019) demonstrate both notably different changes between these two taxa in how shell properties shifted under altered seawater chemistry, as well as cryptic changes that were not obvious by eye.
When Tegula and Nucella were exposed to reduced-pH seawater for 6 months, the former experienced substantial reductions in shell growth (~88%) and strength (~50%), while the latter experienced no effects on growth and only moderate reductions in strength (~10%) (Fig. 4). In this case, shell strength was measured in response to a compressive load applied perpendicular to the axis of coiling in the manner of a crab pinch. The use of intact shells accounted for failure traits that were dependent on both material properties and shell geometry. As has been shown in analogous systems involving biting consumers where gape dimensions are important (e.g. Dumont and Herrel, 2003), the observed patterns in breaking force, and their relationship to shell growth and size, have ecological relevance. Shells of smaller width and height are susceptible to a broader range of size classes of crushing predators, and declines in shell strength decrease the force required of a predator. Therefore, although both gastropod species will likely become increasingly vulnerable to predation under future ocean conditions, Tegula may become substantially more so. At the same time, crustaceans that often dominate guilds of shell-crushing predators, including species such as the common rock crabs, Cancer productus and Romaleon antennarium, appear less susceptible to OA (Ries et al., 2009; Whiteley, 2011). Such differential responses to shifts in seawater chemistry could skew biotic interactions involving various combinations of predators and prey (Kroeker et al., 2014b).
Figure 4.
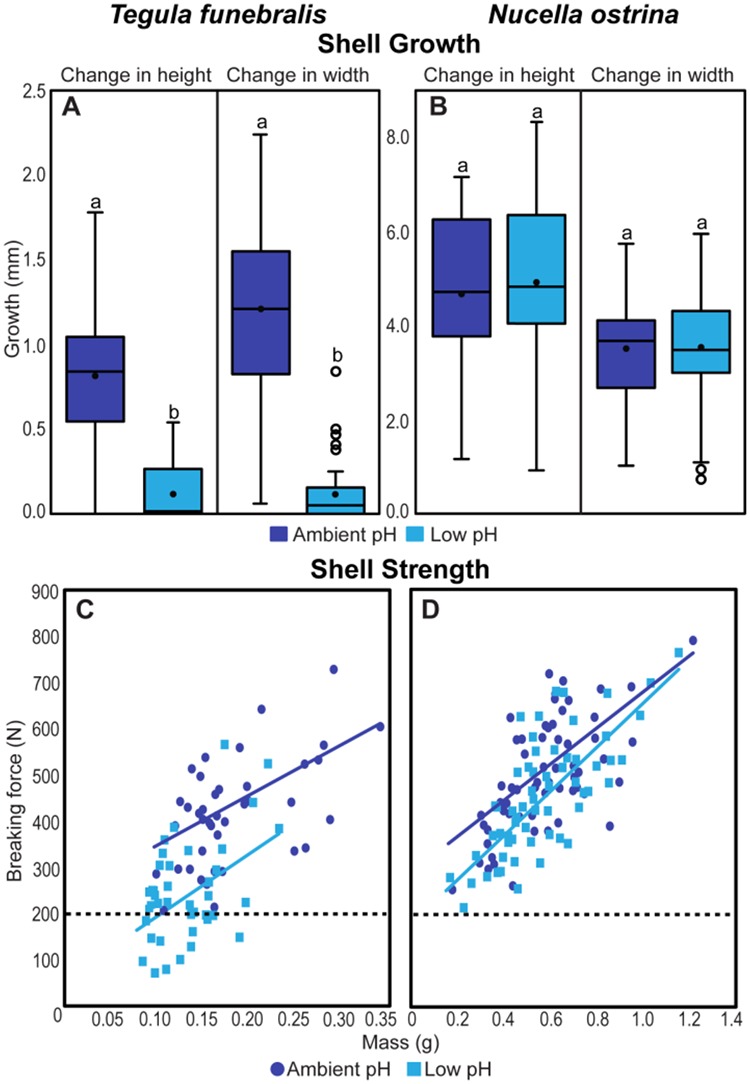
Shell growth and strength of the black turban snail, T. funebralis, and the striped dogwhelk, N. ostrina after 185 days of exposure to decreased seawater pH (pH ~ 7.4, half a unit lower on the total scale than ambient seawater). Note that the axes differ between left and right panels given the different growth and strength patterns of Tegula (left) and Nucella (right). (A and B) Boxplots of new shell growth (both height and width) measured over the 185-day experiment (n = 40 per treatment). Ambient treatments are in dark blue, manipulated (decreased) pH treatments are in light blue, with boxes indicating upper and lower quartiles, central lines indicating medians, means as black circles, and whiskers representing min/max data. (C and D) Scatterplots of shell strength (n = 20 and 30 per treatment for Tegula and Nucella, respectively). Mass (g) of dried shells was used as a proxy for size. Maximum force (N) values indicate forces exerted at the point of total shell failure. Dark blue (ambient pH) and light blue (low pH) trend lines indicate best-fit regressions. The black-dotted line at 200 N indicates conservative crushing forces exerted by adult crabs of C. productus (Taylor, 2000). Modified from Barclay et al. (2019).
In addition to shifts in shell size and/or shape, changes in mineralogy and/or microstructure could contribute to shell weakening. In the case of Tegula and Nucella, these two species precipitate shells that differ in composition. The former has a nacreous shell made of columnar/stacked aragonite crystals, whereas the latter has an inner cross-lamellar aragonitic shell with an outer layer of homogeneous calcite (Geller, 1982; Avery and Etter, 2006). Calcite is chemically more stable than aragonite and less prone to dissolution (Watabe, 1988). The potential for divergent mineralogies to lead to distinct responses to OA is well recognized (e.g. Welladsen et al., 2010; Ries, 2011; Amaral et al., 2012; Coleman et al., 2014; MacKenzie et al., 2014; Wright et al., 2014, 2018; Moulin et al., 2015; Pickett and Andersson, 2015; Dery et al., 2017). Still, because mineralogical responses to shifts in temperature or OA may not be visually obvious, and yet have the capacity to affect shell integrity (and therefore resistance to predation), explicit examinations of shell and skeletal structural properties remain important. Indeed, had Barclay et al. (2019) not included a mechanical test of shell strength, one might have assumed that Nucella’s anti-predator defenses were unaffected by OA, given that its macroscopic patterns of growth did not change in low-pH seawater.
The case study of Tegula and Nucella also underscores additional ways in which environmental change might shift the character of predator–prey interactions. Although further experiments are required, the disproportionate decline in growth and shell strength in Tegula under OA implies a potential for crabs to alter how they rank this species as a food source relative to Nucella. Both gastropods are frequently prey for shell-crushing crabs, but under normal conditions, Tegula is more resistant to predation than similarly sized Nucella. In particular, crabs attacking Tegula are more likely to fail in the attack, and the handling times for Tegula are significantly greater. Nevertheless, crabs regularly target Tegula, suggesting that black turban snails are preferred for other reasons (e.g. they may contain more tissue mass for a given size). If such is the case, then under future ocean conditions when breaking strengths of Tegula are likely to decline to levels readily managed by crabs, it is conceivable that crabs will pursue Tegula with substantially greater vigor than Nucella. Because Tegula and Nucella occupy different trophic levels—with the former herbivorous and the latter carnivorous—changes in relative predation pressure could modify patterns of energy flow through the food web. This potential for environmental change to reconfigure feeding links in marine communities warrants further research.
Ocean chemistry, behaviour and altered community structure
One of the more far-reaching implications of OA is that chemical changes to seawater can induce marked shifts in animal behaviour (Munday et al., 2009; Dixson et al., 2010; Nilsson et al., 2012; Watson et al., 2013; Jellison et al., 2016; Jellison and Gaylord, 2019; Rivest et al., 2019). Such shifts in behaviour complement those tied to altered biomechanical properties by influencing foraging activities of organisms and their responses to predators. One example of where OA-induced behavioural impairments may have critical implications is in tidepool systems. Pisaster ochraceus, the ochre sea star, operates as a major predator along rocky shores of the eastern Pacific Ocean, where foundational work by Paine (1969) highlighted its keystone role in influencing community structure through its consumption of the dominant mussel space-holder, M. californianus. However, Pisaster and other predatory sea stars such as the six-armed star, Leptasterias hexactis, have also been shown to influence community organization not only by eating mussels, but also by inducing widespread flight responses and altered foraging patterns in less preferred prey such as herbivorous gastropods. In this regard, factors that affect how prey responds behaviourally to predatory sea stars could perturb community structure and dynamics.
Laboratory trials indicate the capacity for OA to influence refuge-seeking behaviours and foraging activities of the black turban snail, T. funebralis, in response to Pisaster and Leptasterias sea stars (Gooding et al., 2009; Jellison et al., 2016; Jellison and Gaylord, 2019). Tegula is an abundant and conspicuous herbivore along many rocky shores of the US west coast, and operates as a non-trivial contributor to energy transfer through these systems. Therefore, it is of note that Tegula snails held in low-pH seawater typical of intertidal rock pools display attenuated predator-avoidance behaviours. Moreover, such behavioural impairments may become more common as OA exacerbates the low-pH conditions that currently derive from the accumulation of respiratory CO2 in the pools when they are isolated during night-time low tides (Jellison et al., 2016; Kwiatkowski et al., 2016; Silbiger and Sorte, 2018; Jellison and Gaylord, 2019; also see Bracken et al., 2018).
Tegula’s role as a common grazer means additionally that effects of predatory sea stars can extend beyond Tegula to influence trophic levels below; in this case, the macroalgae grazed by snails. Such cascading effects can follow two paths. One arises when the sea stars eat Tegula individuals, which decreases the number of snails and how much macroalgae gets consumed. Another path derives from the propensity of Tegula to behaviourally avoid sea stars, which can reduce foraging by the snails when they detect sea stars (i.e. Tegula becomes too fearful to feed). Both these pathways could be altered by low pH. For example, OA-induced impairment of anti-predator behaviour could make Tegula snails more vulnerable to predation, increasing their mortality rates and decreasing how many remain to feed on macroalgae. Alternatively, OA could cause atypical boldness in snails, attenuating their flight responses and raising their risk of being eaten, but also fostering elevated foraging and per-capita consumption of macroalgae.
Mesocosm experiments used to explore these dual pathways demonstrate that decreased seawater pH can lead to an overall reduction in the cascading influence of sea stars on macroalgal consumption by Tegula snails (Fig. 5; Jellison and Gaylord, 2019). Under contemporary seawater conditions, as snails represent a less preferred prey item for sea stars (Gravem and Morgan, 2019), they are rarely eaten, yet still demonstrate a strong avoidance behaviour and a reduced rate of grazing on macroalgae when the scent of sea stars is present. However, in low-pH seawater, the antipredator behaviour of snails becomes muted, causing them to partially ignore sea-star scent. This outcome results in increased snail feeding relative to that which occurs in ambient seawater containing the aroma of sea stars. Moreover, the increased feeding rates under low-pH conditions are sufficiently elevated that, even though more snails are killed by sea stars, the snails that remain eat more macroalgae overall. By this mechanism, low pH can weaken the positive indirect influence of sea stars on macroalgae.
Figure 5.
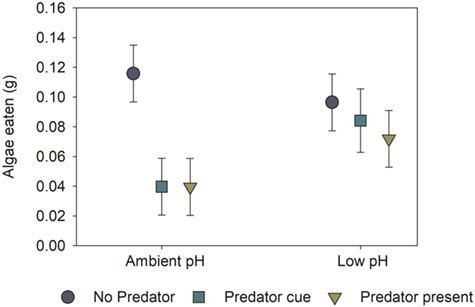
Exposure to low-pH seawater can attenuate the antipredator behaviour of herbivorous snails (T. funebralis) that inhabit tidepools, altering their grazing patterns and thus the way in which cascading, top–down effects of sea-star predators operate in the system. Under ambient conditions (pH ~ 7.9 on the total scale) in mesocosm experiments, snails exit the water when they sense the presence of sea-star predators (L. hexactis; yellow triangle) or dissolved cue from them (blue–green square); this flight response provides a spatial refuge from predation and reduces the grazing pressure of snails on macroalgae. However, under low-pH conditions (pH ~ 6.9, expected in isolated tidepool waters during nighttime low tides under OA), snails spend more time in the water in the presence of sea stars, increasing their access to and consumption of macroalgae relative to when they are in ambient seawater. Error bars represent 95% confidence intervals. Figure modified from Jellison and Gaylord (2019).
In addition to OA-induced behavioural alterations that impinge on predator–prey interactions, competitive interactions can shift within communities subjected to perturbed seawater chemistry. McCoy and Pfister (2014) demonstrate shifts in the dominance hierarchy of calcified coralline algae that compete through overgrowth interactions on rocky intertidal shores. In particular, these researchers revealed a reorganization between the 1980s and the present day of the rankings that dictate which species is superior to another in this guild. Although additional work is required to understand the underlying drivers of such changes, these modified competitive interactions could be mediated at least in part through properties or processes associated with biomineralization. Analogous but more dramatic shifts in competitive hierarchies occur in response to OA in tropical reef systems. In these latter cases, weedy, mat-forming algae gain prominence at the expense of other taxa such as corals (Fabricius et al., 2011; Harley et al., 2012; Connell et al., 2013).
Biomechanics of heat, momentum and mass exchange under global change
As alluded to above, California mussels appear vulnerable to climatic changes. Consistent with this possibility, the abundance and size of the congeners M. californianus and M. trossulus have declined in northern Washington State, with modelling suggesting a causal link to OA (Wootton et al., 2008). Percent cover of M. californianus in Southern California has fallen relative to historical levels (Smith et al., 2006), and the abundance of another species, M. edulis, on the US east coast has dropped by >60% since the 1970s (Sorte et al., 2017). The latter two studies implicate temperature increases as a possible driver. Long-term research along the Oregon coast, spanning >14 years, has additionally revealed correlations between large-scale oceanographic indices (in particular, the North Pacific Gyre Oscillation, or NPGO) and mussel recruitment (Menge et al., 2009). These correlations suggest a relationship between climate-driven shifts in ocean dynamics and larval survival, potentially due to NPGO-related changes in phytoplankton food availability. Connections between mussel performance, food and global change factors (including temperature and seawater pH; Juranek et al., 2009; Alin et al., 2012; Davis et al., 2018) are further reiterated by correlations between geographic variation in the strength of coastal upwelling and growth of juvenile mussels. Throughout California and other portions of the US west coast, decreased pH and low food are associated with reduced mussel growth, as are peak aerial temperatures during low-tide emergence (Kroeker et al., 2016). Complementary results from laboratory trials using M. galloprovincialis likewise demonstrate declines in growth under OA, with warmer water temperatures offsetting this trend (Kroeker et al., 2014a). O’Donnell et al. (2013) and Zhao et al. (2017) document negative effects of OA on the attachment strength of the byssal threads that allow mussels to adhere to the rock; such responses could further contribute to population decreases in these species.
Given the ecological status of mussels as major space occupiers and providers of habitat for other species and juvenile conspecifics, their impaired performance under OA and warming has strong implications for a variety of interactions in intertidal environments. In what follows we emphasize interactions governed by physical transport and exchange processes that often operate as foci for biomechanical study, and which underpin the capacity for intra- and inter-specific facilitation by mussels. At low tide, dense beds of adult mussels ameliorate stresses for juvenile size classes and other organisms that live within the mussel assemblage. In particular, thermal and desiccation stresses, as controlled by heat transfer and evaporative mass exchange, can be modulated appreciably within M. californianus beds, such that temperature and desiccation extremes are substantially attenuated (Jurgens and Gaylord, 2016, 2018). Indeed, thermal extremes are so effectively buffered in the midst of mussel aggregations that typical latitudinal patterns in high-temperature exposure are functionally eliminated (Jurgens and Gaylord, 2018). Reduction of temperature and desiccation stress enables residents of mussel beds to tolerate low-tide temperature variability under current climate conditions, but also hints at the potential for a dramatic tipping point to become problematic in the future. For example, cascading biodiversity loss could occur if previously noted declines in mussel abundance or density (Smith et al., 2006; Sorte et al., 2017) dip below thresholds necessary for them to support their own recruitment or the success of resident taxa (Jurgens and Gaylord, 2018; see also Sunday et al., 2017).
Although facilitative effects often dominate the ecological role of mussels, physical effects of high-biomass species aggregations can also vary markedly in space and time. In certain habitat locations, otherwise strongly facilitative species can exacerbate climate-related heat and desiccation stresses. For example, evaluations of the thermal mechanics of the system indicate that aerial temperatures at low tide on the surface of mussel beds can exceed those on adjacent bedrock, likely due to the dark colour and discrete size of the shells that encourages strong solar absorption while also limiting the extent to which heat conducts away into deeper portions of the rock (Jurgens and Gaylord, 2016). Were small size classes of juvenile and sub-adult mussels to position themselves on the bed surface, they would routinely experience heat and desiccation stresses in excess of their thermal tolerances (Fig. 6; Jurgens and Gaylord, 2016). However, the smallest size classes of mussels tend to inhabit the interiors of mussel beds, where conditions are more amenable to survival (Fig. 6). Quantitative understanding of physical feedbacks between climate-driven stressors and within-habitat conditions relevant to associated organisms are therefore critical for predicting population impacts of climate change that stem from physiological stresses (Fig. 6; Jurgens and Gaylord, 2016, 2018).
Figure 6.
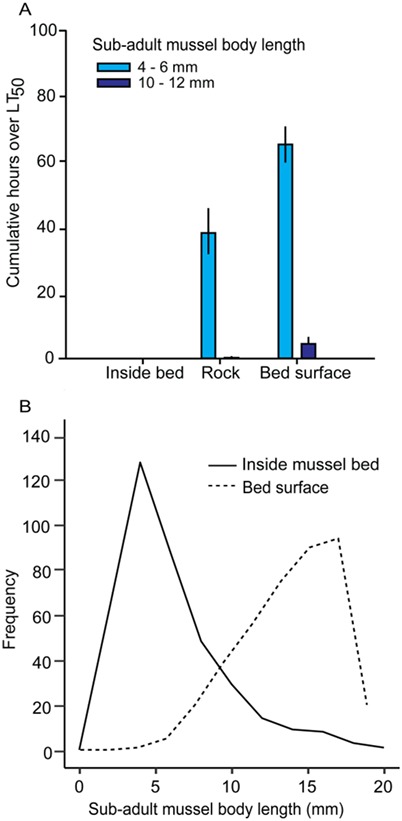
Biophysical interactions can alleviate or exacerbate climate-related stresses for the many taxa that occupy biogenic habitats, but climate risk depends on physiology (e.g. size-specific thermal tolerance) and microhabitat use. (A) Cumulative duration of temperatures over lethal tolerances (here, LT50; the temperature at which 50% of animals die) for two size classes of sub-adult mussels (Mytilus californianus) in biogenic (interior and surface locations of mussel beds) and abiotic (rock clearing) habitats. Conditions commonly exceed lethal thermal tolerances for small sub-adults, and occasionally for larger, more heat-tolerant individuals. Data are from 15 months (1 June 2012 to 1 September 2013) of 30-min temperature measurements on horizontal surfaces (loggers: Maxim® DS-1921-G iButtons; n = 4 per microhabitat; calibrated, waterproofed in parafilm and coated with marine epoxy). (B) Frequency distribution of sub-adult mussels by size (2-mm bins) as found in the field in within-bed and surface-bed microhabitats at a site in northern California, USA over 1 year (N = first 100 individuals sampled per location once per season; seasons pooled). Since larger sub-adults tend to live at the bed surface (B), as climate change exacerbates current conditions, these size classes are more likely to experience major mortality events (despite relatively higher thermal tolerance) than smaller individuals living inside the well-buffered bed. Further experimental details and full dataset available in Jurgens and Gaylord (2016).
It is additionally clear that a perspective focused exclusively on low-tide conditions would overlook other factors that can be important for shoreline creatures. In the case of organisms that reside in the interstices of mussel beds, conditions at high tide are strikingly different from those during periods of aerial emergence. This point raises the question of how decreased exchange of mass and momentum might influence chemical parameters of seawater within the interiors of submerged mussel beds. Two types of chemical perturbation have special relevance. First, organisms like mussels and other heterotrophic animals that reside within mussel beds consume dissolved oxygen, making it less available for themselves and other species. Second, the release of CO2 associated with respiration and calcification processes (or CO2 uptake during photosynthesis by autotrophs; Hurd, 2000; Cornwall et al., 2015) can alter the carbonate system of seawater, exacerbating or potentially offsetting (e.g. Koweek et al., 2018) the consequences of human-produced CO2 absorbing into the oceans.
Thus, although attenuated heat and mass exchange to and from the interior of mussel beds may ameliorate thermal and desiccation stresses for resident organisms at low tide, and although analogous reductions in momentum flux at high tide may provide shelter from large hydrodynamic forces that characterize rocky intertidal habitats (e.g. Denny, 1995; Gaylord, 1999, 2000; Gaylord et al., 2008; Jensen and Denny, 2016), tradeoffs can arise. In particular, elevated chemical stresses can manifest within the interstices of dense aggregations of marine organisms during immersion. For example, we measured seawater conditions in the gaps between mussels within an intact bed transplanted into a flow tank from the field, and did so across a range of flow conditions external to the assemblage. At seawater velocities near zero, the respiration of mussels comprising a bed can decrease oxygen concentrations by approximately 10% (25 μmol kg−1) relative to control seawater (Fig. 7; Ninokawa et al., 2019). Likewise, respiration and calcification can lower seawater pH by 0.1 unit compared to surrounding waters. This latter modification corresponds to a decline in the saturation state of calcium carbonate—a factor influencing the ability of many marine calcifiers to produce shells and skeletons (Ries, 2011)—of over 0.25 units (Fig. 7). Note that these chemical changes are applicable to mussel beds in habitats continually refreshed with new seawater, and could be much greater for mussel aggregations within tidepools or other restricted water bodies. The changes in pH and saturation state are also comparable in magnitude to those observed for the open ocean since the preindustrial period. Therefore, seawater conditions within the interstices of immersed mussel beds may cross thresholds for calcification and other physiological processes decades sooner than expected based on projections for the ocean more broadly. The chemical alterations to seawater within the close confines of mussel beds arise most strongly when fluid-dynamic mixing processes are slow, highlighting the importance of ambient flow conditions and interactions between hydrodynamics and the physical structure of the bed. Higher seawater velocities and associated shear and vertical mixing (see, e.g. Gaylord et al., 2004, 2012) will increase rates of water exchange into and out of a mussel bed and will tend to homogenize the interior and exterior seawater chemistry. Likewise, gradients between the interior and exterior of the bed will depend on factors such as the size and packing density of mussels, which also influence how readily mixing occurs. An exploration of such details awaits further attention.
Figure 7.
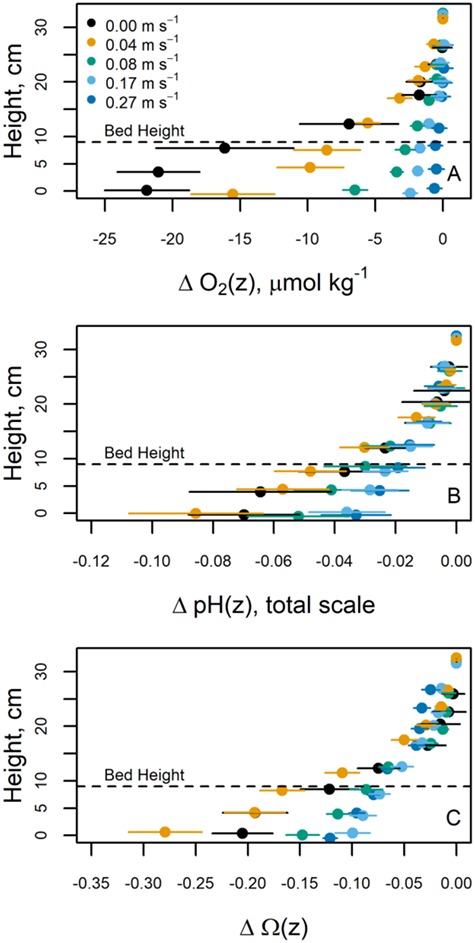
Seawater chemistry can be altered within a mussel bed. Vertical difference profiles for (A) dissolved oxygen, (B) pH (total scale) and (C) calcite saturation state, Ω, across a range of water velocities external to the bed (various colors). Delta symbol indicates differences between chemical parameters at height, z (measured upward from the base of the mussel bed), and their corresponding values at the top of each profile. All of these chemical parameters are depressed inside the bed compared to the bulk seawater. Dashed lines indicate the height of the experimental mussel bed. Error bars depict the standard error across multiple profiles within each flow bin. Data have been jittered in the y-axis for visibility. Figure modified from Ninokawa et al. (2019).
Summary
The global environment is changing in response to human activities, as evidenced by trends of warming and acidification in the world’s oceans. These shifting conditions have consequences for biomechanical function and behaviour of organisms, together with cascading implications for how individuals and species interact with one another in communities. Although just a first step towards adequate understanding, recent studies of marine taxa on temperate rocky shores reveal the capacity for—and to some extent the scope of—future changes that can be expected due to ocean warming and acidification within these vital ecosystems.
Acknowledgements
We thank T. Hill, E. Sanford, P. Shukla, G. Ng, K. Elsmore, A. Smart, A. Saley, A. Ricart and S. Mendonca for helpful discussions and early input regarding ideas that appear in this work.
Funding
This work was supported by the National Science Foundation (OCE-1636191 to BG) and the National Science and Engineering Research Council of Canada (2015-04244 to LRL). B.M.J. and A.T.N. were additionally funded by National Science Foundation Graduate Research Fellowships. K.M.B. received a Vanier Canadian Graduate Scholarship (CSG) and a Michael Smith Foreign Study Supplement.
References
- Abbott IA, Hollenberg GJ (1976) Marine Algae of California. Stanford University Press, Stanford [Google Scholar]
- Alin SR, Feely RA, Dickson AG, Hernandez-Ayon JM, Juranek LW, Ohman MD, Goericke R (2012) Robust empirical relationships for estimating the carbonate system in the southern California current system and application to CalCOFI hydrographic cruise data (2005–2011). J Geophys Res 117: C05033. doi: 10.1029/2011JC007511. [DOI] [Google Scholar]
- Amaral V, Cabral H, Bishop MJ (2012) Effects of estuarine acidification on predator-prey interactions. Mar Ecol Prog Ser 445: 117–127. [Google Scholar]
- Avery R, Etter RJ (2006) Microstructural differences in the reinforcement of a gastropod shell against predation. Mar Ecol Prog Ser 323: 159–170. [Google Scholar]
- Barclay KM, Gaylord B, Jellison BM, Shukla P, Sanford E, Leighton LR (in press) Marked variation in the effects of ocean acidification on shell growth and strength in two intertidal gastropods. Mar Ecol Prog Ser. [Google Scholar]
- Bellwood DR, Hoey AS, Ackerman JL, Depczynski M (2006) Coral bleaching, reef fish community phase shifts and the resilience of coral reefs. Glob Chang Biol 112: 1587–1594. [Google Scholar]
- Bracken MES, Silbiger NJ, Bernatchez G, Sorte CJB (2018) Primary producers may ameliorate impacts of daytime CO2 addition in a coastal marine ecosystem. PeerJ 6: e4739. doi: 10.7717/peerj.4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno JF, Stachowicz JJ, Bertness MD, (2003) Inclusion of facilitation into ecological theory. Trends Ecol Evol 18: 119–125. [Google Scholar]
- Byrne M, Przeslawski R (2013) Multistressor impacts of warming and acidification of the ocean on marine invertebrates' life histories. Integr Comp Biol 53: 582–596. [DOI] [PubMed] [Google Scholar]
- Caldeira K, Wickett ME (2003) Anthropogenic carbon and ocean pH. Nature 425: 365–365. [DOI] [PubMed] [Google Scholar]
- Carlton JT. (ed) (2007) The Light and Smith manual: Intertidal Invertebrates from Central California to Oregon. University of California Press, Berkeley [Google Scholar]
- Chan F, Chan F, Barth JA, Blanchette CA, Byrne RH, Chavez F, Cheriton O, Feely RA, Friederich G, Gaylord B, Gouhier T, et al. (2017) Persistent spatial structuring of coastal ocean acidification in the California current system. Sci Rep 7: 2526. doi: 10.1038/s41598-017-02777-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman DW, Byrne M, Davis AR (2014) Molluscs on acid: gastropod shell repair and strength in acidifying oceans. Mar Ecol Prog Ser 509: 203–211. [Google Scholar]
- Connell JH. (1961) The influence of interspecific competition and other factors on the distribution of the barnacle Chthamalus stellatus. Ecology 42: 710–723. [Google Scholar]
- Connell SD, Kroeker KJ, Fabricius KE, Kline DI, Russell BD (2013) The other ocean acidification problem: CO2 as a resource among competitors for ecosystem dominance. Philos Trans R Soc Lond B Biol Sci 368. doi: 10.1098/rstb.2012.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall CE, Revill AT, Hurd CL (2015) High prevalence of diffusive uptake of CO2 by macroalgae in a temperate subtidal ecosystem. Photosynth Res 124: 181–190. [DOI] [PubMed] [Google Scholar]
- Davis CV, Hewett K, Hill TM, Largier JL, Gaylord B, Jahncke J (2018) Reconstructing aragonite saturation state based on an empirical relationship for northern California. Estuar Coasts 41: 2056–2069. [Google Scholar]
- Dayton PK. (1971) Competition, disturbance, community organization: the provision and subsequent utilization of space in a rocky intertidal community. Ecol Monogr 41: 351–389. [Google Scholar]
- Dayton PK. (1972) Toward an understanding of community resilience and the potential effects of enrichment to the benthos at McMurdo Sound, Antarctica In Parker BC, ed, Proceedings of the Colloquium on Conservation Problems in Antarctica. Allen Press, Lawrence, Kansas [Google Scholar]
- Denny M. (1995) Predicting physical disturbance – mechanistic approaches to the study of survivorship on wave-swept shores. Ecol Monogr 65: 371–418. [Google Scholar]
- Denny MW. (2016) Ecological Mechanics: Principles of Life’s Physical Interactions. Princeton University Press, Princeton. [Google Scholar]
- Denny MW, Gaylord B (2010) Marine ecomechanics. Annu Rev Mar Sci 2: 89–114. [DOI] [PubMed] [Google Scholar]
- Dery A, Collard M, Dubois P (2017) Ocean acidification reduces spine mechanical strength in Euechinoid but not in Cidaroid Sea urchins. Environ Sci Technol 51: 3640–3648. [DOI] [PubMed] [Google Scholar]
- Dixson DL, Munday PL, Jones GP (2010) Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol Lett 13: 68–75. [DOI] [PubMed] [Google Scholar]
- Doney SC, Fabry VJ, Feely RA (2009) Ocean acidification: the other CO2 problem. Annu Rev Mar Sci 1: 169–192. [DOI] [PubMed] [Google Scholar]
- Dumont ER, Herrel A (2003) The effects of gape angle and bite point on bite force in bats. J Exp Biol 206: 2117–2123. [DOI] [PubMed] [Google Scholar]
- Estes JA, Terborgh J, Brashares JS, Power ME, Berger J, Bond WJ, Carpenter SR, Essington TE, Holt RD, Jackson JBC, et al. (2011) Trophic downgrading of planet earth. Science 333: 301–306. [DOI] [PubMed] [Google Scholar]
- Fabricius KE, Langdon C, Uthicke S, Humphrey C, Noonan S, De’ath G, Okazaki R, Muehllehner N, Glas MS, Lough JM (2011) Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat Clim Chang 1: 165–169. [Google Scholar]
- Feely RA, Sabine CL, Lee K, Berelson W, Kleypas J, Fabry VJ, Millero FJ (2004) Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305: 362–366. [DOI] [PubMed] [Google Scholar]
- Feely RA, Sabine CL, Hernandez-Ayon JM, Iason D, Hales B (2008) Evidence for upwelling of corrosive “acidified” water onto the continental shelf. Science 320: 1490–1492. [DOI] [PubMed] [Google Scholar]
- Feely RA, Alin S, Carter B, Bednarsek B, Hales B, Chan F, Hill T, Gaylord B, Sanford E, Byrne RH, et al. (2016) Chemical and biological impacts of ocean acidification along the west coast of North America. Estuar Coast Shelf Sci 183: 260–270. [Google Scholar]
- Frieder CA, Gonzalez JP, Bockmon EE, Navarro MO, Levin LA (2014) Can variable pH and low oxygen moderate ocean acidification outcomes for mussel larvae? Glob Chang Biol 20: 754–764. [DOI] [PubMed] [Google Scholar]
- Gaylord B. (1999) Detailing agents of physical disturbance: wave-induced velocities and accelerations on a rocky shore. J Exp Mar Biol Ecol 239: 85–124. [Google Scholar]
- Gaylord B. (2000) Biological implications of surf-zone flow complexity. Limnol Oceanogr 45: 174–188. [Google Scholar]
- Gaylord B, Gaines SD (2000) Temperature or transport? Range limits in marine species mediated solely by flow. Am Nat 155: 769–789. [DOI] [PubMed] [Google Scholar]
- Gaylord B, Reed DC, Washburn L, Raimondi PT (2004) Physical-biological coupling in spore dispersal of kelp forest macroalgae. J Mar Sys 49: 19–39. [Google Scholar]
- Gaylord B, Reed DC, Raimondi PT, Washburn L (2006) Macroalgal spore dispersal in coastal environments: mechanistic insights revealed by theory and experiment. Ecol Monogr 76: 481–502. [Google Scholar]
- Gaylord B, Denny MW, Koehl MAR (2008) Flow forces on seaweeds: field evidence for roles of wave impingement and organism inertia. Biol Bull 215: 295–308. [DOI] [PubMed] [Google Scholar]
- Gaylord B, Hill TM, Sanford ED, Lenz EA, Jacobs LA, Sato KN, Russell AD, Hettinger A (2011) Functional impacts of ocean acidification in an ecologically critical foundation species. J Exp Biol 214: 2586–2594. [DOI] [PubMed] [Google Scholar]
- Gaylord B, Nickols KJ, Jurgens L (2012) Roles of transport and mixing processes in kelp forest ecology. J Exp Biol 215: 997–1007. [DOI] [PubMed] [Google Scholar]
- Gaylord B, Kroeker KJ, Sunday JM, Anderson KM, Barry JP, Brown NE, Connell SD, Dupont S, Fabricius KE, Hall-Spencer JM, et al. (2015) Ocean acidification through the lens of ecological theory. Ecology 96: 3–15. [DOI] [PubMed] [Google Scholar]
- Geller JB. (1982) Microstructure of shell repair materials in Tegula funebralis (Adams, Al, 1855). Veliger 25: 155–159. [Google Scholar]
- Gooding RA, Harley CDG, Tang E (2009) Elevated water temperature and carbon dioxide concentration increase the growth of a keystone echinoderm. Proc Natl Acad Sci U S A 106: 9316–9321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin LA, Qian P-Y (1997) Juvenile mortality in benthic marine invertebrates. Mar Ecol Prog Ser 146: 265–282. [Google Scholar]
- Gravem SA, Morgan SG (2019) Trait-mediated indirect effects in a natural tidepool system. Mar Biol 166: 1–16. [Google Scholar]
- Harley CDG, Hughes AR, Hultgren KM, Miner BG, Sorte CJB, Thornber CS, Rodriguez LF, Tomanek L, Williams SL (2006) The impacts of climate change in coastal marine systems. Ecol Lett 9: 228–241. [DOI] [PubMed] [Google Scholar]
- Harley CDG, Anderson KM, Demes KW, Jorve JP, Kordas RL, Coyle TA, Graham MH (2012) Effects of climate change on global seaweed communities. J Phycol 48: 1064–1078. [DOI] [PubMed] [Google Scholar]
- Harvell CD, Montecino-Latorre D, Caldwell JM, Burt JM, Bosley K, Keller A, Heron SF, Salomon AK, Lee L, Pontier O, et al. (2019) Disease epidemic and a marine heat wave are associated with the continental-scale collapse of a pivotal predator (Pycnopodia helianthoides). Sci Adv 5: eaau7042. doi: 10.1126/sciadv.aau7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmuth BS, Harley CDG, Halpin P, O’Donnell M, Hofmann GE, Blanchette C (2002) Climate change and latitudinal patterns of intertidal thermal stress. Science 298: 1015–1017. [DOI] [PubMed] [Google Scholar]
- Helmuth B, Broitman BR, Blanchette CA, Gilman S, Halpin P, Harley CDG, O’Donnell MJ, Hofmann GE, Menge B, Strickland D (2006) Mosaic patterns of thermal stress in the rocky intertidal zone: implications for climate change. Ecol Monogr 76: 461–479. [Google Scholar]
- Hoffman GE, Todgham AE (2010) Living in the now: physiological mechanisms to tolerate a rapidly changing environment. Annu Rev Physiol 72: 127–145. [DOI] [PubMed] [Google Scholar]
- Hurd CL. (2000) Water motion, marine macroalgal physiology, and production. J Phycol 36: 453–472. [DOI] [PubMed] [Google Scholar]
- Jackson JBC, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, Bourque BJ, Bradbury RH, Cooke R, Erlandson J, Estes JA, et al. (2001) Historical overfishing and the recent collapse of coastal ecosystems. Science 293: 629–638. [DOI] [PubMed] [Google Scholar]
- Jellison BM, Gaylord B (2019) Shifts in seawater chemistry disrupt trophic links within a simple shoreline food web. Oecologia 190: 955–967. doi: 10.1007/s00442-019-04459-0. [DOI] [PubMed] [Google Scholar]
- Jellison BM, Ninokawa AT, Hill TM, Sanford E, Gaylord B (2016) Ocean acidification alters the response of intertidal snails to a key sea star predator. Proc R Soc B 283: 20160890. doi: 10.1098/rspb.2016.0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MM, Denny MW (2016) Life in an extreme environment: characterizing wave-imposed forces in the rocky intertidal zone using high temporal resolution hydrodynamic measurements. Limnol Oceangr 61: 1750–1761. [Google Scholar]
- Jones CG, Lawton JH, Shachak M (1994) Organisms as ecosystem engineers. Oikos 69: 373–386. [Google Scholar]
- Juranek LW, Feely RA, Peterson WT, Alin SR, Hales B, Lee K, Sabine CL, Peterson J (2009) A novel method for determination of aragonite saturation state on the continental shelf of Central Oregon using multi-parameter relationships with hydrographic data. Geophys Res Lett 36: L24601. doi: 10.1029/2009GL040778. [DOI] [Google Scholar]
- Jurgens LJ, Gaylord B (2016) Edge effects reverse facilitation by a widespread foundation species. Sci Rep 6: 37573. doi: 10.1038/srep37573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens LJ, Gaylord B (2018) Physical effects of habitat-forming species override latitudinal trends in temperature. Ecol Lett 21: 190–196. [DOI] [PubMed] [Google Scholar]
- Koweek DA, Zimmerman RC, Hewett KM, Gaylord B, Giddings SN, Nickols KJ, Ruesink JL, Stachowicz JJ, Takeshita Y, Caldeira K (2018) Expected limits on the ocean acidification buffering potential of a temperate seagrass meadow. Ecol Appl 28: 1694–1714. [DOI] [PubMed] [Google Scholar]
- Kroeker KJ, Kordas RL, Crim RN, Singh GG (2010) Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol Lett 13: 1419–1434. [DOI] [PubMed] [Google Scholar]
- Kroeker KJ, Fiorenza M, Gambi MC (2013a) Ocean acidification causes ecosystem shifts via altered competitive interactions. Nat Clim Chang 3: 156–159. [Google Scholar]
- Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, Singh GS, Duarte CM, Gattuso JJP (2013b) Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob Chang Biol 19: 1884–18896. doi: 10.1111/gcb.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeker KJ, Gaylord B, Hill TM, Hosfelt JD, Miller SH, Sanford E (2014a) The role of temperature in determining species' vulnerability to ocean acidification: a case study using Mytilus galloprovincialis. PLoS One 9: e100353. doi: 10.1371/journal.pone.0100353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeker KJ, Sanford E, Jellison BM, Gaylord B (2014b) Predicting the effects of ocean acidification on predator-prey interactions: a conceptual framework based on coastal molluscs. Biol Bull 226: 211–222. [DOI] [PubMed] [Google Scholar]
- Kroeker KJ, Sanford E, Rose JM, Blanchette CA, Chan F, Chavez FP, Gaylord B, Helmuth B, Hill TM, Hofmann GE, et al. (2016) Interacting environmental mosaics drive geographic variation in mussel performance and predation vulnerability. Ecol Lett 19: 771–779. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski L, Gaylord B, Hill T, Hosfelt J, Kroeker KJ, Nebuchina Y, Ninokawa A, Russell A, Rivest EB, Sesboue M, et al. (2016) Nighttime dissolution in a temperate coastal ocean ecosystem increases under acidification. Sci Rep 6: 22984. doi: 10.1038/srep22984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty KD, Suchanek TH (2016) Revisiting Paine’s 1966 sea star removal experiment, the most-cited empirical article in the American naturalist. Am Nat 188: 365–378. [DOI] [PubMed] [Google Scholar]
- Ling SD. (2008) Range expansion of a habitat-modifying species leads to loss of taxonomic diversity: a new and impoverished reef state. Oecologia 156: 883–894. [DOI] [PubMed] [Google Scholar]
- Ling SD, Scheibling RE, Rassweiler A, Johnson CR, Shears N, Connell SD, Salomon SK, Norderhaug KM, Perez-Matus A, Hernandez JC, et al. (2015) Global regime shift dynamics of catastrophic sea urchin overgrazing. Phil Trans R Soc B 370: 20130269. doi: 10.1098/rstb.2013.0269. [DOI] [Google Scholar]
- Lubchenco J. (1978) Plant species diversity in a marine intertidal community—importance of herbivore food preference and algal competitive abilities. Am Nat 112: 23–39. [Google Scholar]
- Mackenzie CL, Ormondroyd GA, Curling SF, Ball RJ, Whiteley NM, Malham SK (2014) Ocean warming, more than acidification, reduces shell strength in a commercial shellfish species during food limitation. PLoS One 9: e86764. doi: 10.1371/journal.pone.0086764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy SJ, Pfister CA (2014) Historical comparisons reveal altered competitive interactions in a guild of crustose coralline algae. Ecol Lett 17: 475–483. [DOI] [PubMed] [Google Scholar]
- McCoy SJ, Kamenos NA, Chung P, Wootton TJ, Pfister CA (2018) A mineralogical record of ocean change: decadal and centennial patterns in the California mussel. Glob Chang Biol 24: 2554–2562. [DOI] [PubMed] [Google Scholar]
- Menge BA, Sutherland JP (1987) Community regulation: variation in disturbance, competition, and predation in relation to environmental stress and recruitment. Am Nat 130: 730–757. [Google Scholar]
- Menge BA, Chan F, Nielsen KJ, DiLorenzo E, Lubchenco J (2009) Climatic variation alters supply-side ecology: impact of climate patterns on phytoplankton and mussel recruitment. Ecol Monogr 79: 379–395. [Google Scholar]
- Miller LP. (2013) The effect of water temperature on drilling and ingestion rates of the dogwhelk Nucella lapillus feeding on Mytilus edulis mussels in the laboratory. Mar Biol 160: 1489–1496. [Google Scholar]
- Morris RH, Abbott DP, Haderlie EC (1980) Intertidal Invertebrates of California. Stanford University Press, Stanford [Google Scholar]
- Moulin L, Grosjean P, Leblud J, Batigny A, Collard M, Dubois P (2015) Long-term mesocosms study of the effects of ocean acidification on growth and physiology of the sea urchin Echinometra mathaei. Mar Environ Res 103: 103–114. [DOI] [PubMed] [Google Scholar]
- Munday PL, Dixson DL, Donelson JM, Jones GP, Pratchett MS, Devitsina GV, Doving KB (2009) Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc Natl Acad Sci U S A 106: 1848–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelkerken I, Connell SD (2015) Global alteration of ocean ecosystem functioning due to increasing human CO2 emissions. Proc Natl Acad Sci U S A 112: 13272–13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickols KJ, White JW, Largier JL, Gaylord B (2015) Marine population connectivity: reconciling large-scale dispersal and high self-retention. Am Nat 185: 196–211. [DOI] [PubMed] [Google Scholar]
- Nilsson GE, Dixson DL, Domenici P, McCormick MI, Sorensen C, Watson SA, Munday PL (2012) Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nat Clim Chang 2: 201–204. [Google Scholar]
- Ninokawa AT, Takeshita Y, Jellison BM, Jurgens LJ, Gaylord B (2019) Biological modification of seawater chemistry by an ecosystem engineer, the California mussel, Mytilus californianus. Limnol Oceanogr. 1–16 doi: 10.1002/lno.11258. [DOI] [Google Scholar]
- O’Connor MI, Bruno JF, Gaines SD, Halpern BS, Lester SE, Kinlan BP, Weiss JM (2007) Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proc Natl Acad Sci U S A 104: 1266–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell MJ, George MN, Carrington E (2013) Mussel byssus attachment weakened by ocean acidification. Nat Clim Chang 3: 587–590. [Google Scholar]
- Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, Feely RA, Gnanadesikan A, Gruber N, Ishida A, Joos F, et al. (2005) Anthropogenic Ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437: 681–686. [DOI] [PubMed] [Google Scholar]
- Paine RT. (1966) Food web complexity and diversity. Am Nat 100: 65–75. [Google Scholar]
- Paine RT. (1969) A note on trophic complexity and community stability. Am Nat 103: 91–93. [Google Scholar]
- Pfister CA, Roy K, Wootton JT, McCoy SJ, Paine RT, Suchanek TH, Sanford E (2016) Historical baselines and the future of shell calcification for a foundation species in a changing ocean. Proc R Soc B 283: 20160392. doi: 10.1098/rspb.2016.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett M, Andersson AJ (2015) Dissolution rates of biogenic carbonates in natural seawater at different pCO(2) conditions: a laboratory study. Aquat Geochem 21: 459–485. [Google Scholar]
- Poloczanska ES, Brown CJ, Sydeman WJ, Kiessling W, Schoeman DS, Moore PJ, Brander K, Bruno JF, Buckley LB, Burrows MT, et al. (2013) Global imprint of climate change on marine life. Nat Clim Chang 3: 919–925. [Google Scholar]
- Power ME, Tilman D, Estes JA, Menge BA, Bond WJ, Mills LS, Daily G, Castilla JC, Lubchenco J, Paine RT (1996) Challenges in the quest for keystones. Bioscience 46: 609–620. [Google Scholar]
- Reguero BG, Losada IJ, Mendez FJ (2019) A recent increase in global wave power as a consequence of oceanic warming. Nat Commun 10: 205. doi: 10.1038/s41467-018-08066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries JB. (2011) Skeletal mineralogy in a high-CO2 world. J Exp Mar Biol Ecol 403: 54–64. [Google Scholar]
- Ries JB, Cohen AL, McCorkle DC (2009) Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37: 1131–1134. [Google Scholar]
- Rivest E, Jellison B, Ng G, Satterthwaite E, Bradley HL, Williams SL, Gaylord B (2019) Mechanisms involving sensory pathway steps inform impacts of global climate change on ecological processes. Front Mar Sci 6: 346. doi: 10.3389/fmars.2019.00346. [DOI] [Google Scholar]
- Sanford E, Gaylord B, Hettinger A, Lenz EA, Meyer K, Hill TM (2014) Ocean acidification increases the vulnerability of native oysters to predation by invasive snails. Proc R Soc B 281: 20132681. doi: 10.1098/rspb.2013.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford E, Sones JL, Garcia-Reyes M, Goddard JHR, Largier JL (2019) Widespread shifts in the coastal biota of northern California during the 2014–2016 marine heatwaves. Sci Rep 9: 4216. doi: 10.1038/s41598-019-40784-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer M, Carpenter S, Foley JA, Folke C, Walker B (2001) Catastrophic shifts in ecosystems. Nature 413: 591–596. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K. (1997) Animal Physiology, EdEd 5 Cambridge University Press, Cambridge, UK [Google Scholar]
- Schultz JA, Cloutier RN, Cote IM (2016) Evidence for a trophic cascade on rocky reefs following sea star mass mortality in British Columbia. PeerJ 4: e1980. doi: 10.7717/peerj.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbiger NJ, Sorte CJB (2018) Biophysical feedbacks mediate carbonate chemistry in coastal ecosystems across spatiotemporal gradients. Sci Rep 8: 796. doi: 10.1038/s41598-017-18736-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JR, Fong P, Ambrose RF (2006) Long-term change in mussel (Mytilus californianus Conrad) populations along the wave-exposed coast of southern California. Mar Biol 149: 537–545. [Google Scholar]
- Somero GN. (2010) The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J Exp Biol 213: 912–920. [DOI] [PubMed] [Google Scholar]
- Sorte CJB, Davidson VE, Franklin MC, Benes KM, Doellman MM, Etter RJ, Hannigan RE, Lubchenco J, Menge BA (2017) Long-term declines in an intertidal foundation species parallel shifts in community composition. Glob Chang Biol 23: 341–352. [DOI] [PubMed] [Google Scholar]
- Sousa WP. (1979) Disturbance in marine intertidal boulder fields: the nonequilibrium maintenance of species diversity. Ecology 60: 1225–1239. [Google Scholar]
- Stachowicz JJ. (2001) Mutualism, facilitation, and the structure of ecological communities. Bioscience 51: 235–246. [Google Scholar]
- Suchanek TH. (1992) Extreme biodiversity in the marine-environment: mussel bed communities of Mytilus californianus. Northwest Environ J 8: 150–152. [Google Scholar]
- Sunday JM, Fabricius KE, Kroeker KJ, Anderson KM, Brown NE, Barry JP, Connell SD, Dupont S, Gaylord B, Hall-Spencer JM, et al. (2017) Ocean acidification can mediate biodiversity shifts by changing biogenic habitat. Nat Clim Chang 7: 81–85. [Google Scholar]
- Taylor GM. (2000) Maximum force production: why are crabs so strong? Proc R Soc B 267: 1475–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeij GJ. (1982) Unsuccessful predation and evolution. Am Nat 120: 701–720. [Google Scholar]
- Vitousek PM, Mooney HA, Lubchenco J, Melillo JM (1997) Human domination of Earth's ecosystems. Science 277: 494–499. [Google Scholar]
- Watabe N. (1988) Shell structure In Trueman ER, Clarke MR, eds, The Mollusca, 11. Form and Function. Academic Press, Plymouth, UK, pp. 69–104. [Google Scholar]
- Watson S-A, Lefevre S, McCormick MI, Domenici P, Nilsson GE, Munday PL (2013) Marine mollusc predator-escape behaviour altered by near-future carbon dioxide levels. Proc R Soc B 281: 20132377 doi: 10.1098/rspb.2013.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welladsen HM, Southgate PC, Heimann K (2010) The effects of exposure to near-future levels of ocean acidification on shell characteristics of Pinctada fucata (Bivalvia: Pteriidae). Molluscan Res 30: 125–130. [Google Scholar]
- Whiteley NM. (2011) Physiological and ecological responses of crustaceans to ocean acidification. Mar Ecol Prog Ser 430: 257–271. [Google Scholar]
- Wootton JT, Pfister CA, Forester JD (2008) Dynamic patterns and ecological impacts of declining ocean pH in a high-resolution multi-year dataset. Proc Natl Acad Sci U S A 48: 18848–18853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JM, Parker LM, O’Connor WA, Williams M, Kube P, Ross PM (2014) Populations of Pacific oysters Crassostrea gigas respond variably to elevated CO2 and predation by Morula marginalba. Biol Bull 226: 269–281. [DOI] [PubMed] [Google Scholar]
- Wright JM, Parker LM, O’Connor WA, Scanes E, Ross PM (2018) Ocean acidification affects both the predator and prey to alter interactions between the oyster Crassostrea gigas (Thunberg, 1793) and the whelk Tenguella marginalba (Blainville, 1832). Mar Biol 165: 46. doi: 10.1007/s00227-018-3302-6. [DOI] [Google Scholar]
- Young IR, Zieger S, Babanin AV (2011) Global trends in wind speed and wave height. Science 332: 451–455. [DOI] [PubMed] [Google Scholar]
- Zhao X, Guo C, Han Y, Che Z, Wang Y, Wang X, Chai X, Wu H, Liu G (2017) Ocean acidification decreases mussel byssal attachment strength and induces molecular byssal responses. Mar Ecol Prog Ser 565: 67–77. [Google Scholar]


