Abstract
Polyketide C-methylation occurs during a programmed sequence of dozens of reactions carried out by multidomain polyketide synthases (PKSs). Fungal PKSs perform these reactions iteratively, where a domain may be exposed to and act upon multiple enzyme-tethered intermediates during biosynthesis. We surveyed a collection of C-methyltransferase (CMeT) domains from non-reducing fungal PKSs to gain insight into how different methylation patterns are installed. Our in vitro results show that control of methylation resides primarily with the CMeT, and CMeTs can intercept and methylate intermediates from non-cognate non-reducing PKS domains. Furthermore, the methylation pattern is likely imposed by a competition between methylation or ketosynthase-catalyzed extension for each intermediate. Understanding site-specific polyketide C-methylation may facilitate targeted C-C bond formation in engineered biosynthetic pathways.
Polyketide synthases (PKSs) have been long-standing targets for engineering biosynthetic pathways because of the inherent structural and stereochemical complexity built into their products.1 PKSs tether growing intermediates to the enzyme by thioesterification to the phosphopantetheine arm of a carrier protein that delivers the intermediates to client domains for successive rounds of elongation and modification. The core reaction—ketosynthase-catalyzed C-C bond formation by decarboxylative Claisen condensation—extends the intermediate by two carbons each cycle. However, PKSs occasionally implement a secondary C-C bond forming reaction through regiospecific α-methylation by an S-adenosylmethionine (SAM)-dependent C-methyltransferase (CMeT) embedded in the PKS.
Type I highly-reducing PKSs (HR-PKSs) found in both marine cyanobacteria and fungi, modular and iterative systems respectively, can methylate polyene β-ketoacyl substrates as in curacin and lovastatin biosynthesis, through use of a PKS-embedded CMeT.2, 3 In both examples, multiple β-ketoacyl species of different chain length could be presented to the CMeT, but only a single regiospecific methylation is observed. Some fungal non-reducing PKSs (NR-PKSs) also contain embedded CMeT domains and are known to methylate linear substrates ranging from C4 to C14 prior to cyclization by the product template domain, with many of these CMeTs acting multiple times during iterative catalysis.4, 5 Tolerance for such different acceptor substrates across all PKS CMeTs may reflect an inherently adaptable active site allowing for substrate flexibility.
Although structures of PKS CMeTs remain few in number, features common to these CMeTs may be advantageous to future efforts to engineer site-specific C-C bond formation. In Type I PKS CMeTs, there is a two-subdomain architecture comprised of an N-terminal cap and C-terminal Rossmann fold, with the active site positioned in a cleft at their interface, a diagnostic indicator that the CMeT is active.3, 4 The CurJ and PksCT CMeT active sites are large and hydrophobic, even though some methylated substrates are as small as acetoacetyl-holo-ACP. Although no acceptor substrates have been observed crystallographically in CMeT active sites, only the β–ketoacyl moiety appears to make specific contacts with binding pocket residues, the catalytic His-Glu dyad.4 Therefore, the N-terminal subdomain and large acceptor binding pockets of PKS CMeTs could modulate selectivity or activity towards non-native substrates.
A collection of differently regiospecific CMeTs could be added to an engineered polyketide biosynthetic pathway to elicit a programmable pattern of methylation. Previous work has leveraged the ability to deconstruct multienzymes into their constituent domains as a way to explore NR-PKS activity. Chain length, cyclization, and product release have been surveyed by domain deconstruction and combinatorial domain swap reactions.6–8 We sought to expand this approach to study fungal polyketide C-methylation.
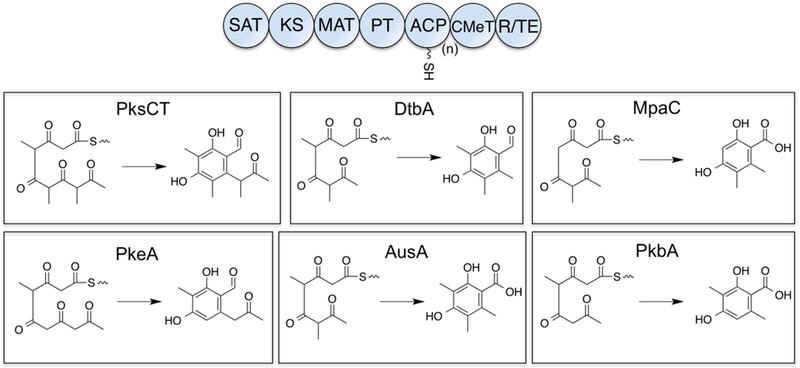
In order to build a domain library for combinatorial swap experiments, we surveyed the literature for fungal Group VI and Group VII NR-PKSs.5 Only those PKSs that had a known product associated with them were included, to allow for comparison of the native methylation pattern to the pattern seen with in vitro domain swap reactions. We omitted PKSs that utilized complex starter units derived from upstream HR-PKSs or FASs such as the asperfuranone PKSs AfoG and AfoE and the alkylisoquinoline synthase PkiA.5, 9 The candidates selected were PksCT (Monascus purpureus)10, MpaC (Penicillium brevicompactum)11, DtbA (Aspergillus niger)12, as well as PkeA, AusA, and PkbA (A. nidulans).5 Each of these PKSs utilizes an acetyl starter unit, produces tetra- or pentaketides, and installs one to three C-methyl groups having differing regiochemistry in the native product, and we reasoned that their similarity would maximize the chances for successful non-cognate interactions (Fig. 1).
Figure 1: Fungal NR-PKSs selected for domain deconstruction and combinatorial swaps.
Group VI and VII NR-PKSs share a common domain architecture (top), with Group VI terminating in a TE domain to release the polyketide as the carboxylic acid and Group VII terminating in a R domain to give an aldehyde. DtbA and PkbA contain tandem ACP domains. (SAT: starter unit-ACP transacylase, KS: ketosynthase, MAT: malonyl-CoA:ACP transacylase, PT: product template, ACP: acyl carrier protein, CMeT: C-methyltransferase, R: reductase, TE: thioesterase).
Using the Udwary-Merski algorithm in combination with the early experience of dissecting PksCT domains4, 13, we generated expression constructs for the SAT-KS-MAT tridomain, and the CMeT and ACP mono-domains (Supplementary Information Table 2). These three proteins would constitute the “minimal methylating PKS,” with all necessary domains to carry out multiple rounds of extension and methylation. Based on previously published PksCT results, we decided to omit PT and R or TE domains and relied on spontaneous intramolecular C-O cleavage of the holo-ACP thioester to release intermediates as pyrones, a commonly observed PKS derailment product.4 This experimental design focused on the known interplay between extension and methylation prior to PT-catalyzed cyclization or R/TE-catalyzed release. Non-methylating fungal NR-PKSs quickly elongate substrates to the full chain-length8, but NR-PKSs with CMeT domains have inherently slower extension activity to allow transit to the ACP-bound intermediates to dock with the CMeT4. Not all desired fragments could be expressed or were soluble, especially for the SAT-KS-MAT fragments; this outcome is consistent with previous attempts to express SAT-KS-MATs across multiple fungal NR-PKS groups and did not improve when alternate cut sites were used.8 Additionally, no fragments from MpaC were soluble. Overall, ACP and CMeT monodomain fragments were generally well behaved for the remaining PKSs in our library.
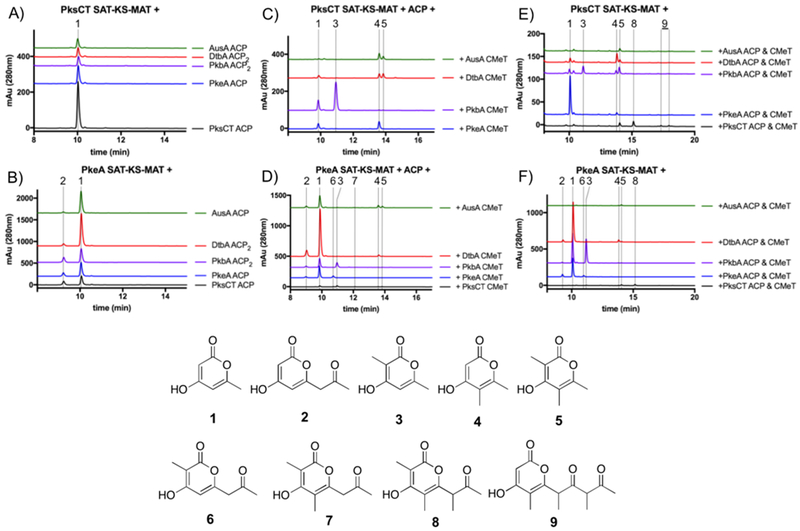
From the soluble, expressed fragments we were able to conduct exploratory reactions to gauge the cross-compatibility of CMeTs with components from other NR-PKSs. All apo-ACPs were activated with Sfp from Bacillus subtilis to give holo-ACP.14 PKS domain fragments were incubated with acetyl-SNAC, malonyl-SNAC, and SAM, and reactions were extracted and analyzed by HPLC and UPLC-ESI-MS. Reconstitution of the minimal PksCT (Figure 2A) was discussed in detail previously4, and the minimal PkeA reaction demonstrated similar behavior (Figure 2B). The major product was the unmethylated triketide 1, however the tetraketide 2 was also detected as a minor product. Thus, the spontaneous release of unmethylated intermediates appears to be a general trend for CMeT-containing NR-PKSs. We speculate that tetraketide 2 may be favored in PkeA because the native substrate is only methylated once following the third extension, so longer unmethylated substrates must be better tolerated for productive biosynthesis.
Figure 2: Reconstitution of the “minimal methylating PKS” for.
A) PksCT minimal reconstitution with non-cognate ACPs. B) PkeA minimal reconstitution with non-cognate ACPs. C) Addition of non-cognate CMeTs to minimal PksCT D) Addition of non-cognate CMeTs to minimal PkeA E) PksCT and F) PkeA SAT-KS-MAT were combined with paired ACP and CMeT. Traces are vertically offset for clarity. See SI Figure 1 and Table 3 for UV/Vis and mass data.
Subsequently, we swapped ACPs to these minimal PKS reactions to determine the compatability of non-cognate ACPs to participate in catalysis. As observed for non-methylating NR-PKSs, ACPs are generally interchangeable, though the yield of pyrone products varied.8 In non-methylating NR-PKSs, dissection generally reduces yield, but the differences in product profiles seen in the above ACP swaps are consistent with previous work.8, 15
We then added CMeT monodomains to PksCT or PkeA minimal PKSs. From the observed pyrones, several aspects of C-methylation were evident. Non-cognate CMeTs generally are capable of intercepting acyl-holo-ACP species and reduce the yield of unmethylated polyketides. Because these pyrones have very similar UV-Vis absorption spectra, we assume peak areas can be roughly correlated. In PksCT-based reactions (Figure 2C), the triketides 3, 4, and 5 accumulate when non-cognate CMeTs are added and are significantly less abundant than 1 in the absence of a CMeT. The position of the methyl group for the singly methylated triketides 3 and 4 has been determined by comparison to an authentic standard of 3.
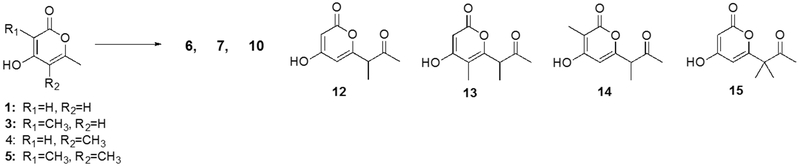
In PkeA-based reactions (Figure 2D), non-cognate CMeTs resulted in the same triketides and additional tetraketides. Notably, the addition of PkeA CMeT to the cognate PkeA SAT-KS-MAT + ACP produced the singly methylated tetraketide 6, consistent with the native PkeA methylation pattern. Because mass fragmentation does not unambiguously reveal the methylation pattern in all cases, we synthesized a series of singly and doubly methylated tetraketides to identify the positions of methyl groups on the detected pyrones. By comparison to these synthetic standards, the position of methyl groups on tetraketides 6 and 7 could be definitively assigned (see Fig. 3). AusA, DtbA, PkbA, and PksCT CMeTs again produced the singly and doubly methylated triketides 3, 4, and 5, although the reduction of 1 seen in Fig. 2C was not as pronounced. This finding could reflect a higher rate of extension by the PkeA KS during the first and second rounds, limiting the chance for methylation of those intermediates. The presence of singly methylated triketide 3 and doubly methylated tetraketide 7 indicate that PksCT CMeT could not always intercept acetoacetyl-holo-ACP following the first extension cycle. The KS domain of PksCT was previously shown to be sensitive to the absence of methylation on the growing intermediate8. Because the native product of PkeA is not methylated following the first two extensions, the KS may be sensitive to the presence of methylation on early stage intermediates, resulting in low overall yield.
Figure 3: Synthetic standards of potential methylation products.
Triacetic acid lactone (1) and variously methylated derivatives 3–5 were obtained by published procedures16, 17 or from commercial sources. These were further elaborated to tetraketides 6, 7, 10, 12–15 as detailed in the Supplementary Information.
A second set of reactions was also carried out, analogous to those above, but the ACP and CMeT were cognate and paired with a non-cognate SAT-KS-MAT (Figure 2E and 2F). In most reactions, the observed products were consistent whether the ACP was cognate to the SAT-KS-MAT or CMeT, with some exceptions. For PksCT SAT-KS-MAT with paired PkbA ACP and CMeT, triketides 4 and 5 were observed in addition to triketides 1 and 3 seen in Figure 2C. These products could reflect preferential binding to the CMeT resulting in methylation. Paired PkeA ACP and CMeT were less effective at producing methylated pyrones with PksCT SAT-KS-MAT than when the ACP was paired with the tridomain, perhaps because PkeA CMeT does not natively methylate early acyl intermediates, and spontaneous release at the triketide stage is faster than the methylation by PkeA CMeT. PksCT ACP and CMeT generated the late-stage tetra- and pentaketide pyrones 8 and 9, as seen previously.4
In total, this series of observations reflects the in trans nature of acyl-ACP:client domain interactions in domain-dissected reconstitution reactions. The growing acyl-ACP intermediates must leave the KS active site, travel through solvent, and dock within the CMeT active site. That the CMeT lowers the rate of extension so significantly, suggests that it strongly competes with the KS for binding of acyl-ACP intermediates. In non-methylating NR-PKSs, extension is very rapid and the growing poly β-ketone intermediate likely remains shielded in the KS, as almost no products of intermediate chain length are detected.18 The addition of a CMeT obviously slows extension as the population of intermediate acyl-ACP species is now split among one additional competing binding partner.
The methylation program for each CMeT does appear to be intrinsic, even in the context of domain dissection. The non-cognate CMeTs methylate substrates with less fidelity than cognate systems, with a bias towards the native methylation pattern of the CMeT. For example, most of the non-cognate CMeTs added to minimal PksCT did not universally eliminate production of the unmethylated triketide 1, even though the cognate PksCT CMeT completely eliminates this product.4 Without more domain fragments, especially SAT-KS-MATs, it is unclear how sensitive KS domains might be to alternate methylation patterns. The absence of improperly methylated intermediates in PksCT reconstitution suggests high fidelity to the “programmed” methylation, but not all of the above reaction outcomes are equally informative. The differing processivity of extension between PksCT and PkeA SAT-KS-MAT domains in the absence of CMeTs supports KS-mediated stabilization of intermediates in accord with the observed methylation programming. For example, if methylating acetoacetyl-ACP after the first Claisen condensation renders the intermediate an unfavorable substrate for continued cycles in PkeA, available free holo-ACP will decrease as the diketide is less susceptible to release as no pyrone formation is possible before the triketide is formed.
These experiments also lack the PT and R or TE domains. Little is known about how these domains are affected by the substrate methylation pattern. Sensitivity of the KS and PT to the stereochemistry of methyl groups, is also unknown. Domain dissection certainly increases the likelihood of racemization of a given α-methyl position as the acyl-ACP docks to other domains in trans, a solvent-exposed process. The interdependence of catalytic activity and substrate affinities (including ACP:client domain binding) in iterative PKS domains is linked by co-evolution and will likely remain an unresolved problem in the field until multidomain structures are available.
The molecular basis of programmed methylation patterns is uncertain, but attempts have been made to functionally account for how iterative catalysis operates. Studies on the fungal HR-PKS LovB, from lovastatin biosynthesis, recently probed how acyl-ACP intermediates are appropriately directed towards the next catalytic domain in the programmed sequence.19 A diene triketide is α-methylated by a CMeT prior to ketoreductase (KR)-catalyzed reduction of the β-ketone to the corresponding alcohol. Because the α-position is most acidic before β-reduction, therefore most available for methylation, the CMeT must act first. Like the NR-PKSs discussed above, LovB appears to be sensitive to substrates that are not methylated; in the absence of SAM, intermediates are spontaneously released prematurely as pyrones.2 By providing synthetic substrates, the authors demonstrated that the CMeT has high catalytic efficiency towards only the on-path diene triketide, whereas the KR is less selective and less efficient. The proper order of methylation and reduction is functionally enforced by C-methylation being very fast for only a single intermediate while ketoreduction is slower but acts on a wider range of substrates, consistent with only a single methylation but multiple ketoreductions during synthesis.
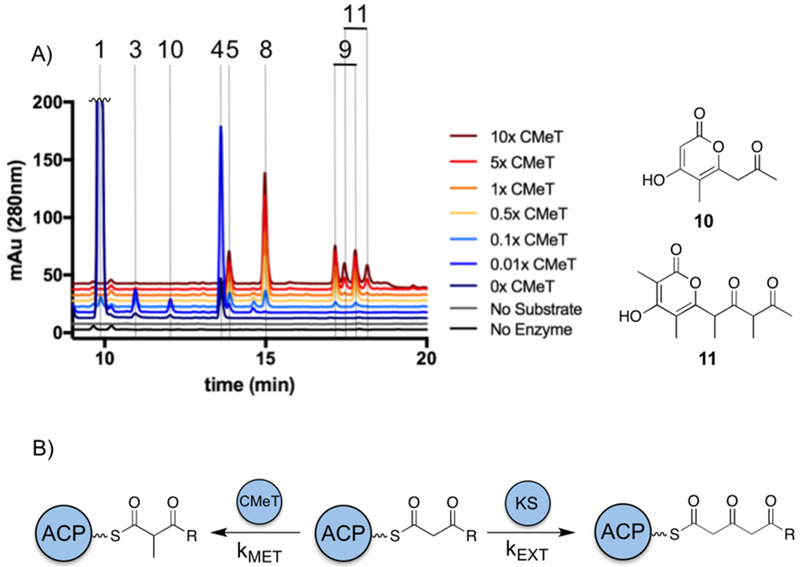
We wanted to probe whether a similar process might be followed in methylating NR-PKSs by balancing rates of extension and methylation. A panel of specific synthetic intermediates could not be screened due to the reactivity of poly-β-ketone intermediates, so therefore the relative amount of CMeT was used to modulate the rate of methylation (Figure 4).
Figure 4: Increasing relative amounts of CMeT results in hypermethylation of pentaketide intermediates.
A) PksCT SAT-KS-MAT and ACP were incubated with increasing concentrations of PksCT CMeT. Traces are vertically stacked and the peak for 1 is truncated for clarity. B) Control of C-methylation programming is proposed to rely on the rates of extension and methylation for each acyl-ACP intermediate produced.
When even very small amounts of CMeT were added to the reaction, the production of triketide lactone 1 was dramatically reduced, and as the relative concentration of CMeT increased the total number of singly methylated pyrones 3, 4, and 10 increased. The methyl position of 10 was determined by comparison to our synthesized standards (see SI for details). At approximately equimolar CMeT, multiply methylated pyrones 5, 8, and 9 dominated the reaction profile. And with excess CMeT, a new pair of product peaks appeared, proposed to be the hypermethylated pentaketide 11. Because 11 elutes as two peaks with identical mass, fragmentation, and chromophores, just like on-path pentaketide 9, it is likely to retain the stereocenters that give rise to diastereomers—demonstrating non-native methylation following the final extension.
We propose that the kinetic balance of extension and methylation determines the observed methylation pattern. As with LovB, different domains may have catalytic efficiency towards different substrates such that only the programmed outcome is kinetically favorable. At low concentrations of CMeT, not all programmed methylations can occur before the KS performs another extension generating an off-path intermediate. At high concentration, the selectivity against positions that are not part of the “methylation program” can be overcome by increasing the overall rate of methylation. These experiments cannot replicate the kinetic advantage found in an intact NR-PKS, where the effective concentrations of acyl-ACP and client domains are high. It is also possible that other conformational changes transmitted within an intact NR-PKS could influence control of methylation. The primary determinant of the methylation pattern, however, appears to be the CMeT domain itself, in addition to surveillance by the KS.
In summary, we assembled a small library of CMeTs and expanded an in vitro domain swap approach to explore the factors controlling polyketide C-methylation. We found that NR-PKS CMeTs were capable of methylating acyl-ACP intermediates in trans, and that the methylation program is primarily intrinsic to the CMeT. The product profiles could be modulated based on the relative concentration of CMeT, even allowing for non-native methylation. Together the data suggest that programmed methylation is a kinetic balance between the rates of extension and methylation, although a molecular or structural basis for differential rates remains to be established. A more comprehensive collection of CMeT domains, development of chimeric intact NR-PKSs, and structures of multidomain PKS fragments with bound substrates will offer further insight into this complex multidomain choreography.
Methods
All reagents were purchased from Sigma Aldrich except 4-hydroxy-3,6-dimethyl-2H-pyran-2-one (3) which was purchased from Acros, AcSNAC, which was synthesized, and MalSNAC, which was prepared enzymatically by MatB and purified.7, 8 Standard molecular biology procedures were used for isolation of DNA and the creation of expression plasmids. Sequencing was performed at the Johns Hopkins University Synthesis and Sequencing Facility. M. purpureus NRRL 1596, A. niger NRRL 328, A. nidulans NRRL 194, and P. brevicompactum NRRL 2011 were received from USDA ARS and cultured on PDA plates. Collected mycelia were flash frozen, ground under N2(l) and gDNA was purified using the DNeasy Plant Mini kit (Qiagen). Overlap extension polymerase chain reaction was used to amplify exons and assemble intron-free expression inserts, which were ligated into pET-24a or pET-28a (Novagen) with N- and/or C-terminal His6 tags.
Details of expression constructs and domain boundaries can be found in the Supplemental Information. Plasmids were transformed to E. coli BL21(DE3) by electroporation and maintained on LB agar plates. Expression cultures were grown in LB media at 37 °C to OD600 of 0.6. Cultures were cold-shocked in an ice bath for about 1 h, and induced with 1 mM IPTG (GoldBio) overnight at 18 °C, shaking at 225 rpm. Cell pellets were harvested by centrifugation for 15 min, at 4,000 x g, 4 °C and either flash frozen in N2(l) and stored at −80 °C until use, or resuspended immediately in 5 mL g−1 cell pellet in lysis buffer (50 mM potassium phosphate, 300 mM NaCl, 10 % (v/v) glycerol, pH 7.6). Resuspended cells were lysed by sonication on ice for 10 x 10 s at 40% amplitude (Vibra-Cell Ultrasonic Processor, Sonics & Materials, Inc.). The lysate was cleared by centrifugation for 25 min at 27,000 x g, 4 °C and batch bound to Co2+-TALON (Clontech) at 4 °C for 1 h. Expressed proteins were purified by gravity column chromatography as follows: the protein-bound resin was washed with lysis buffer (10 CV), followed by lysis buffer + 2 mM imidazole (5 CV) and elution with lysis buffer + 100 mM imidazole (5 CV). Domain fragments were dialyzed into reaction buffer (100 mM potassium phosphate, 5 % (v/v) glycerol, pH 7.0), concentrated by diafiltration, and used immediately or flash frozen in N2(l) and stored at −80 °C.
Protein concentration was determined by Bradford assay (BioRad, Hercules, CA) in duplicate using bovine serum albumin as a standard. Prior to in vitro reactions, ACPs (50 μM) were activated by Sfp (1 μM) with CoASH (100 μM) and MgCl2 (10 mM) in reaction buffer for 1 h at room temperature as previously reported.8 Reconstitution reactions contained 10 μM of each included domain with 0.5 mM AcSNAC, 2 mM MalSNAC, 2 mM SAM, and 1 mM TCEP in reaction buffer totaling 250 μL. After 4 h at room temperature, the reactions were quenched with 5 μL concentrated HCl and extracted into ethyl acetate 3 x 250 μL. The combined organic extracts were dried to a residue and resuspended in 250 μL of 20 % (v/v) aqueous acetonitrile. Extracts were analyzed on an Agilent 1200 HPLC with autosampler by injecting 100 μL onto a Prodigy ODS3 column (4.5 x 250 mm, 5μ, Phenomenex, Torrence, CA) with 5-85 % (v/v) MeCN/H2O, with 0.1 % (v/v) formic acid, over 40 min at 1 mL min−1 and monitored at 280 nm. Mass spectrometric analysis was done using a Waters Acquity Xevo G-2 UPLC-ESI-MS in positive ion mode, with 5 μL injected on a BEHC C18 column and 10-90 % (v/v) MeCN/H2O, with 0.1 % (v/v) formic acid, over 10 min.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health grants RO1 ES001670 and T32 GM080189. We also thank I. P. Mortimer (JHU) for UPLC-MS support.
Footnotes
Supporting Information
Details of cloning, characterization of enzymatic products, syntheses of standards to identify tri- and tetraketides, associated structural characterizations, comparisons and 1H- and 13C-NMR spectra are provided in the Supporting Information. This information is available free of charge via the internet at http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- 1.Staunton J; Weissman KJ, Polyketide biosynthesis: a millennium review. Nat. Prod. Rep 2001, 18, 380–416. [DOI] [PubMed] [Google Scholar]
- 2.Ma SM; Li JW; Choi JW; Zhou H; Lee KK; Moorthie VA; Xie X; Kealey JT; Da Silva NA; Vederas JC; Tang Y, Complete reconstitution of a highly reducing iterative polyketide synthase. Science 2009, 326, 589–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skiba MA; Sikkema AP; Fiers WD; Gerwick WH; Sherman DH; Aldrich CC; Smith JL, Domain Organization and Active Site Architecture of a Polyketide Synthase C-methyltransferase. ACS Chem. Biol 2016, 11, 3319–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Storm PA; Herbst DA; Maier T; Townsend CA, Functional and Structural Analysis of Programmed C-Methylation in the Biosynthesis of the Fungal Polyketide Citrinin. Cell Chem. Biol. 2017, 24, 316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahuja M; Chiang YM; Chang SL; Praseuth MB; Entwistle R; Sanchez JF; Lo HC; Yeh HH; Oakley BR; Wang CC, Illuminating the diversity of aromatic polyketide synthases in Aspergillus nidulans. J. Am. Chem. Soc 2012, 134, 8212–8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crawford JM; Thomas PM; Scheerer JR; Vagstad AL; Kelleher NL; Townsend CA, Deconstruction of iterative multidomain polyketide synthase function. Science 2008, 320, 243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vagstad AL; Newman AG; Storm PA; Belecki K; Crawford JM; Townsend CA, Combinatorial Domain Swaps Provide Insights into the Rules of Fungal Polyketide Synthase Programming and the Rational Synthesis of Non-Native Aromatic Products. Angew. Chem. Int. Ed. Engl 2013, 52, 1718–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman AG; Vagstad AL; Storm PA; Townsend CA, Systematic domain swaps of iterative, nonreducing polyketide synthases provide a mechanistic understanding and rationale for catalytic reprogramming. J. Am. Chem. Soc 2014, 136, 7348–7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang YM; Szewczyk E; Davidson AD; Keller N; Oakley BR; Wang CC, A gene cluster containing two fungal polyketide synthases encodes the biosynthetic pathway for a polyketide, asperfuranone, in Aspergillus nidulans. J. Am. Chem. Soc 2009, 131, 2965–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu T; Kinoshita H; Ishihara S; Sakai K; Nagai S; Nihira T, Polyketide synthase gene responsible for citrinin biosynthesis in Monascus purpureus. Appl. Environ. Microbiol 2005, 71, 3453–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Regueira TB; Kildegaard KR; Hansen BG; Mortensen UH; Hertweck C; Nielsen J, Molecular basis for mycophenolic acid biosynthesis in Penicillium brevicompactum. Appl. Environ. Microbiol 2011, 77, 3035–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh H; Chang SL; Chiang YM; Bruno KS; Oakley BR; Wu TK; Wang CC, Engineering Fungal Nonreducing Polyketide Synthase by Heterologous Expression and Domain Swapping. Org. Lett 2013, 15, 756–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Udwary DW; Merski M; Townsend CA, A method for prediction of the locations of linker regions within large multifunctional proteins, and application to a type I polyketide synthase. J. Mol. Biol 2002, 323, 585–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quadri LE; Weinreb PH; Lei M; Nakano MM; Zuber P; Walsh CT, Characterization of Sfp, a Bacillus subtilis phosphopantetheinyl transferase for peptidyl carrier protein domains in peptide synthetases. Biochemistry 1998, 37, 1585–1595. [DOI] [PubMed] [Google Scholar]
- 15.Huitt-Roehl CR; Hill EA; Adams MM; Vagstad AL; Li JW; Townsend CA, Starter unit flexibility for engineered product synthesis by the nonreducing polyketide synthase PksA. ACS Chem. Biol 2015, 10, 1443–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokoe H; Mitsuhashi C; Matsuoka Y; Yoshimura T; Yoshida M; Shishido K, Enantiocontrolled total syntheses of breviones A, B, and C. J. Am. Chem. Soc 2011, 133, 8854–8857. [DOI] [PubMed] [Google Scholar]
- 17.Leiris SJ; Khdour OM; Segerman ZJ; Tsosie KS; Chapuis JC; Hecht SM, Synthesis and evaluation of verticipyrone analogues as mitochondrial complex I inhibitors. Bioorg. Med. Chem 2010, 18, 3481–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vagstad AL; Bumpus SB; Belecki K; Kelleher NL; Townsend CA, Interrogation of global active site occupancy of a fungal iterative polyketide synthase reveals strategies for maintaining biosynthetic fidelity. J. Am. Chem. Soc 2012, 134, 6865–6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cacho RA; Thuss J; Xu W; Sanichar R; Gao Z; Nguyen A; Vederas JC; Tang Y, Understanding Programming of Fungal Iterative Polyketide Synthases: The Biochemical Basis for Regioselectivity by the Methyltransferase Domain in the Lovastatin Megasynthase. J. Am. Chem. Soc 2015, 137, 15688–15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.