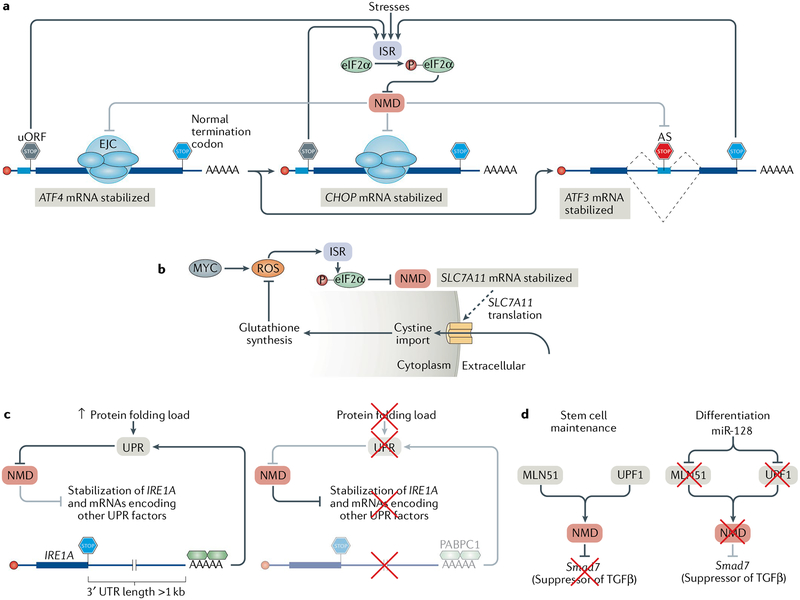
Fig. 5 |. Physiological roles of nonsense-mediated mRNA decay.
a | Various extracellular stresses can induce the integrated stress response (ISR). During the ISR, eukaryotic initiation factor 2α (eIF2α) is phosphorylated (P), leading to the suppression of nonsense-mediated mRNA decay (NMD). NMD suppression enables the expression of NMD targets, including those encoding the transcription factors activating transcription factor 4 (ATF4), CCAAT-enhancer-binding protein homologous protein (CHOP) and ATF3, which coordinate the expression of proteins that alleviate the stresses. Following resolution of the ISR, NMD is resumed and suppresses the expression of ATF4 and CHOP, because their mRNAs contain an upstream open reading frame (uORF), and of ATF3, because of a premature termination codon introduced by alternative splicing (AS), thereby ensuring that the ISR is only active during stress. b | Cells that constitutively express the oncogene MYC produce toxic reactive oxygen species (ROS), which activate the ISR and cause eIF2α phosphorylation and NMD suppression, thereby stabilizing SLC7A11 mRNA. SLC7A11 encodes a transporter that imports cystine into the cytoplasm, which is used in the synthesis of the ROS scavenging molecule glutathione. c | Cells with increases in protein-folding demand in the endoplasmic reticulum (ER) induce the unfolded protein response (UPR) in order to increase the folding capacity of the ER (left). The UPR inhibits NMD, and as a result, NMD targets, such as the IRE1A mRNA, which has an unusually long 3′ untranslated region (3′ UTR), are stabilized. IRE1α together with the products of many other natural NMD targets then coordinates the UPR. When the protein-folding capacity of the ER matches demand (right), NMD is active and degrades the IRE1A mRNA and other mRNAs encoding key UPR regulators, thereby ensuring that innocuous stresses do not activate the UPR. d | NMD maintains the pluripotency of mouse neural stem cells by targeting Smad7 mRNA, which encodes a negative regulator of TGFβ signalling. During neural differentiation, the brain-specific microRNA miR-128 is expressed and inhibits the exon junction complex (EJC) component metastatic lymph node 51 (MLN51) and UPF1. This suppresses NMD, enables the production of SMAD7 and facilitates differentiation.