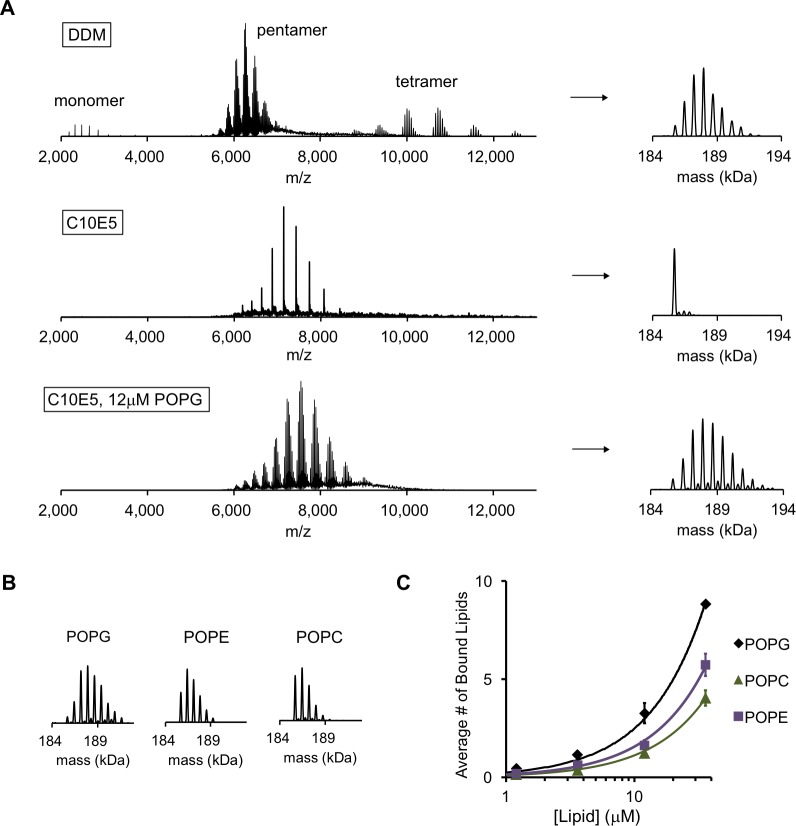
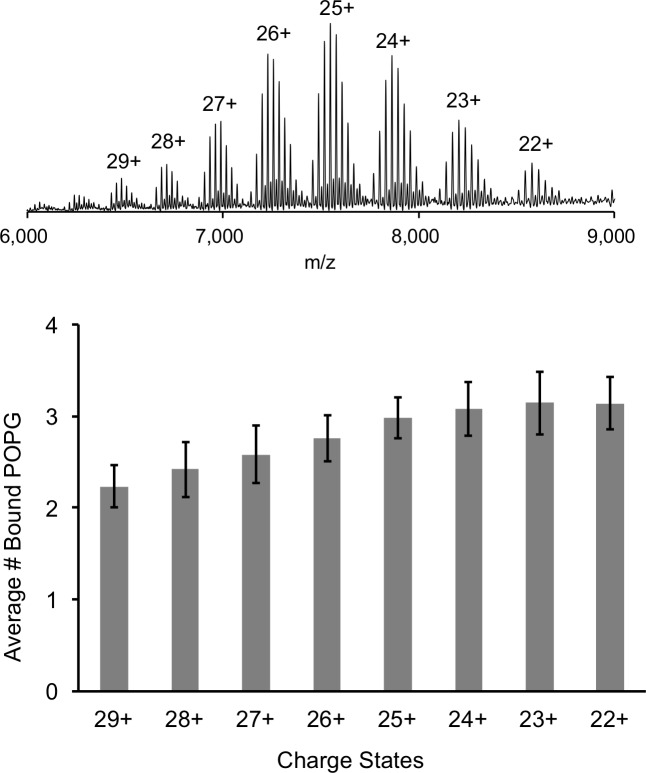
Figure 1. POPG binds selectively to ELIC.
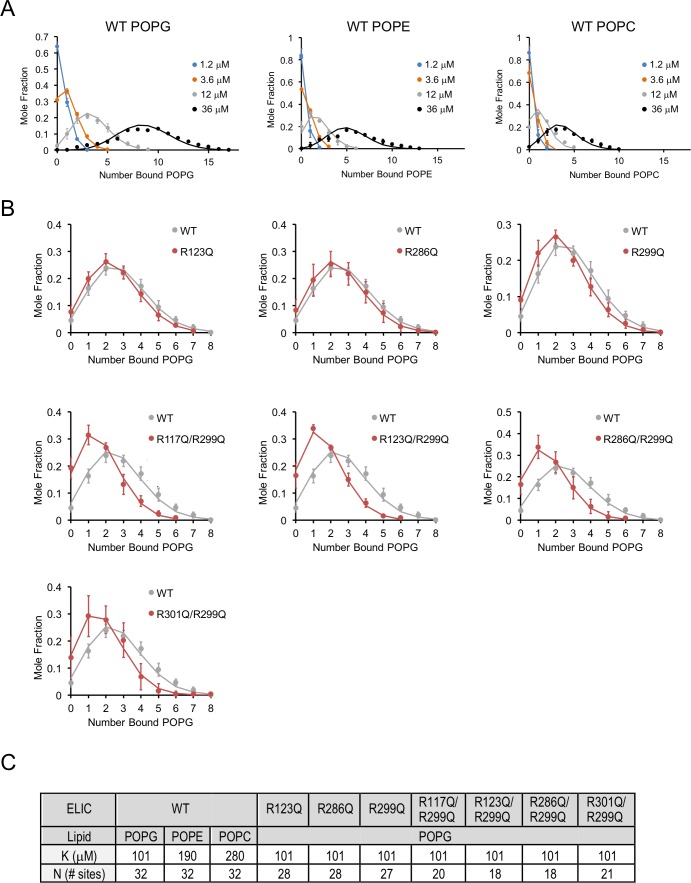
(A) Native MS spectra of ELIC in DDM, C10E5, and C10E5 with 12 μM POPG. Left: full spectra; right: deconvoluted spectra. (B) Deconvoluted spectra of ELIC in 12 μM of the indicated phospholipid. (C) Plot of the average number of bound phospholipids per pentamer at varying concentrations of POPG, POPE, and POPC (n = 3–6, ± SD).
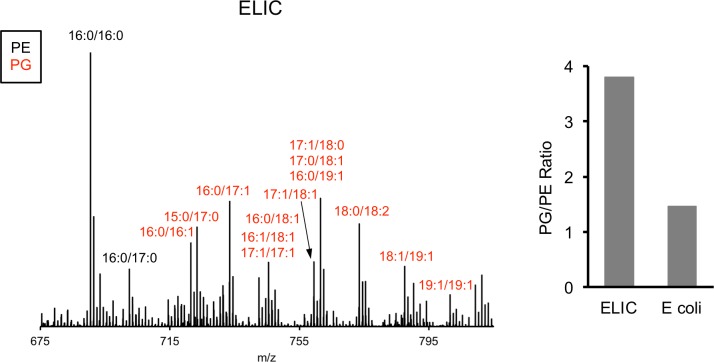
Figure 1—figure supplement 1. MS1 spectra of lipid extract from purified ELIC in DDM and E. coli membranes.
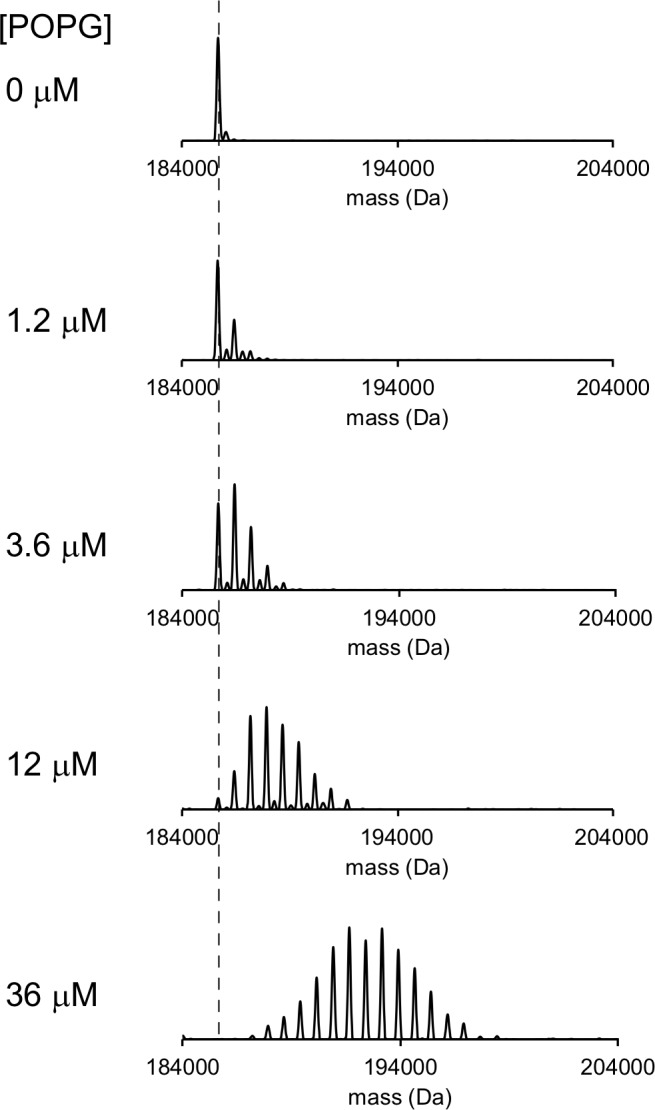
Figure 1—figure supplement 2. Representative deconvoluted spectra of 1 μM ELIC in C10E5 with increasing concentration of POPG.