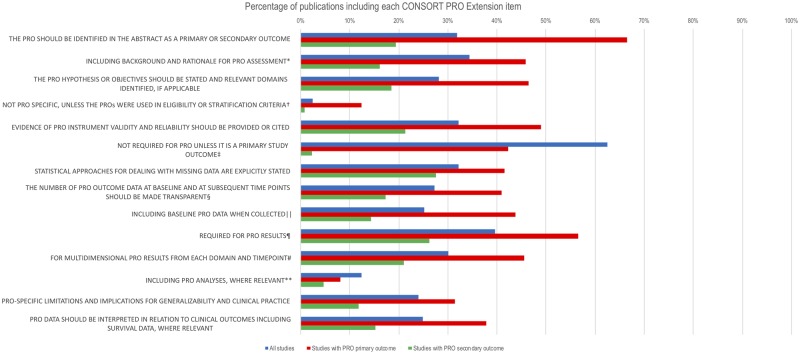
Figure 3.
Percentage of publications (n = 99) including each Consolidated Standards of Reporting Trials (CONSORT) patient-reported outcomes (PRO) Extension Checklist item (adjusted for denominator variation). *PRO elaboration to CONSORT checklist item 2a: “Scientific background and explanation of rationale.” †PRO elaboration to CONSORT checklist item 4a: “Eligibility criteria for participants.” ‡PRO elaboration to CONSORT checklist item 7a: “How sample size was determined.” §PRO elaboration to CONSORT checklist item 13a: “For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analyzed for the primary outcome.” ‖PRO elaboration to CONSORT checklist item 15: “A table showing baseline demographic and clinical characteristics for each group.” ¶PRO elaboration to CONSORT checklist item 16: “For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups.” #PRO elaboration to CONSORT checklist item 17a: “For each primary and secondary outcome, results for each group, the estimated effect size, and its precision (such as 95% confidence interval).” **PRO elaboration to CONSORT checklist item 18: “Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory.”