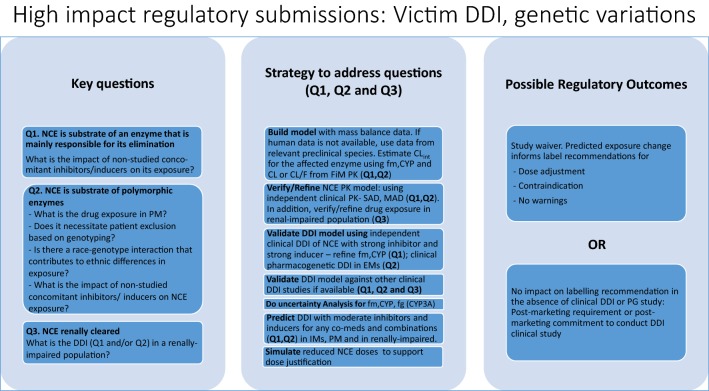
Box 1.
Key questions (Q), modelling strategies and possible outcomes for high-impact regulatory submissions: NCE as a perpetrator of DDI. DDI drug–drug interaction, NCE new chemical entity, CLint intrinsic clearance, CYP cytochrome P450, EM extensive metabolizers, IM intermediate metabolizers, fg fraction escaping intestinal loss, fm,CYP fraction metabolized by an isoform, FiM first in man, SAD single ascending dose, MAD multiple ascending dose, PK pharmacokinetics, PG pharmacogenomic, PM poor metabolizer, CL clearance, CL/F apparent clearance