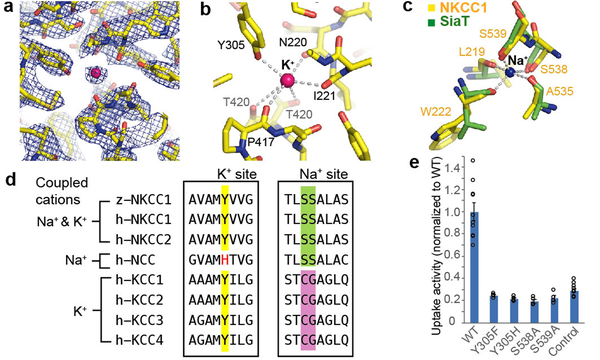
Figure 3 |. Potassium- and sodium-binding sites.
a, Potassium-binding site density map (10.0 σ, blue mesh). Strong extra density is attributed to K+ (pink sphere). b, Potassium-binding site. Dashed lines denote possible coordination. c, Sodium-binding site. NKCC1’s proposed Na+-binding site (yellow) is superimposed onto SiaT’s Na2 site (green). Dashed lines denote the sodium coordination in SiaT. d, The sequence alignment around key K+- or Na+-coordinating residues. Human and zebrafish proteins are denoted by “h” and “z”, respectively. Highlighted positions show Y305 (potassium-coordinating), S538 and S539 (sodium-coordinating). e, Uptake activities of the substrate-binding pocket mutants, normalized to WT (mean±s.e.m., n=4 independent experiments; WT and control are the same as in Fig. 1e).