Abstract
Dorsal, a member of the nuclear factor-kappa B (NF-κB) family of transcription factors, is a critical downstream component of the Toll pathway that regulates the expression of antimicrobial peptides (AMPs) against pathogen invasion. In this study, the full-length ORF of Dorsal was identified from the RNA-seq database of the mealworm beetle Tenebrio molitor (TmDorX2). The ORF of TmDorX2 was 1,482 bp in length, encoding a polypeptide of 493 amino acid residues. TmDorX2 contains a conserved Rel homology domain (RHD) and an immunoglobulin-like, plexins, and transcription factors (IPT) domain. TmDorX2 mRNA was detected in all developmental stages, with the highest levels observed in 3-day-old adults. TmDorX2 transcripts were highly expressed in the adult Malpighian tubules (MT) and the larval fat body and MT tissues. After challenging the larvae with Staphylococcus aureus and Escherichia coli, the TmDorX2 mRNA levels were upregulated 6 and 9 h post infection in the whole body, fat body, and hemocytes. Upon Candida albicans challenge, the TmDorX2 mRNA expression were found highest at 9 h post-infection in the fat body. In addition, TmDorX2-knockdown larvae exposed to E. coli, S. aureus, or C. albicans challenge showed a significantly increased mortality rate. Furthermore, the expression of 11 AMP genes was downregulated in the gut and fat body of dsTmDorX2-injected larvae upon E. coli challenge. After C. albicans and S. aureus challenge of dsTmDorX2-injected larvae, the expression of 11 and 10 AMPs was downregulated in the gut and fat body, respectively. Intriguingly, the expression of antifungal transcripts TmTenecin-3 and TmThaumatin-like protein-1 and -2 was greatly decreased in TmDorX2-silenced larvae in response to C. albicans challenge, suggesting that TmDorX2 regulates antifungal AMPs in the gut in response to C. albicans infection. The AMP expression profiles in the fat body, hemocytes, gut, and MTs suggest that TmDorX2 might have an important role in promoting the survival of T. molitor larvae against all mentioned pathogens.
Subject terms: Antimicrobial responses, Innate immunity, RNAi
Introduction
Insects are confronted by a variety of complex and evolving pathogens, and require a sophisticated and strong innate immune system because they lack adaptive immunity1. Besides primary defenses, that include integument and gut epithelium barriers, the insect innate immune system comprises cellular and humoral defense responses2. Humoral immunity, which includes Toll and immune deficiency (Imd) signaling pathways, regulates the synthesis of potent antimicrobial peptides (AMPs)3.
The Toll and Imd signaling pathways are triggered once immune-related tissues (e.g., the fat body) sense pathogen-associated molecular patterns (PAMPs) through pathogen recognition receptors (PRRs)4,5. The interaction of PAMPs with PRRs activates the intracellular Toll and Imd signal cascades leading to nuclear translocation of nuclear factor kappa-light-chain-enhancer of activated immune cells (NF-κB) transcription factors such as dorsal and dorsal-related immunity factor (Dif) and Relish for the Toll and Imd pathways, respectively. Ultimately, NF-κB in association with other factors regulate the transcription of AMP genes6,7.
Proteins in the NF-κB family share a strikingly well-conserved structure called the Rel homology domain (RHD), which is known to be involved in DNA binding, dimerization, nuclear localization, and interaction with the inhibitor κB (IκB)8. Based on the presence of multiple copies of ankyrin repeats (ANK) or transactivation domains (TAD) at the C-terminus of RHD, NF-κB proteins are classified into Class I and II, respectively.
Dorsal, a class II NF-κB protein, has been identified in different insects such as beetles9, flies10, mosquitoes11,12, bees13, and silk worms14. Dorsal (14 kb) was initially identified as maternal transcript essential for the embryonic establishment of dorsal-ventral polarization10, and was later described to engage in the immune response against infection by Gram-positive bacteria and fungi15. In Aedes aegypti larvae and female mosquitoes, levels of the Dorsal homolog, AaREL1, are markedly increased after Enterobacter cloacae, Micrococcus luteus, and Beauveria bassiana challenge11. Dorsal silencing experiments have shown that the Toll pathway regulates immune responses against Gram-positive (Paenibacillus larvae) and Gram-negative bacteria (E. coli) infection in honey bee (Apis mellifera) pupae13.
In addition to its function in insects, Dorsal expression and its role in innate immunity has been noted in crustacean species. In the Chinese shrimp (Fenneropenaeus chinensis), antibacterial responses to Micrococcus lysodeikticus (Gram-positive bacteria) and Vibrio anguillarum (Gram-negative bacteria) were mediated by FcDorsal16, whereas in the Pacific white shrimp (Litopenaeus vannamei), LvDorsal regulates the penaeidin-4 gene (antifungal and anti-Gram positive bacterial AMP)17,18. Experiments in the crab model Eriocheir sinensis, showed that EsDorsal was primarily expressed in hemocytes and engaged in antifungal and antibacterial immune responses19. Furthermore, in aquatic invertebrates such as the cyclopoid copepod, Paracyclopina nana, Dorsal and Dorsal-like genes show immune function against stressful environmental conditions20.
The mealworm beetle Tenebrio molitor (Coleoptera) is a reliable model to study host-pathogen interactions during bacterial21–23 and fungal24 infection. In the last decade or so, extracellular events in the T. molitor Toll signaling pathway have been addressed in detail25,26. Contrary to its mammalian counterparts, Toll requires the endogenous ligand spätzle (Spz) for AMP production in insects such as T. molitor, Drosophila, and Manduca sexta26–28. The functional form of Spz is formed when the activated Spz-processing enzyme (SPE) cleaves the pro-protein Spz (pro-Spz). At the last step of the protease cascade, Spzinteracts with the ectodomain of the transmembrane receptor29,30. A recent study has shown that Toll-like receptor 7 from T. molitor (TmToll-7) has anti-E. coli activity inducing expression of antibacterial AMPs31. In fact, we have identified nine extracellular ligands, namely TmSpz-1b, −3, −4, −5, −6, −7, −7a, −7b, 7b and -like (unpublished data). However, the ligand-binding partner for TmToll-7 remains to be elucidated. Furthermore, biochemical studies have shown that lysine-type peptidoglycan (Lys-type PGN) is recognized by the Tenebrio peptidoglycan recognition protein (PGRP-SA/Gram-negative binding protein (GNBP-1) complex32. Moreover, Gram-negative binding protein 3 (TmGNBP3) plays a pivotal role in the induction of TmTenecin-1 after Beauveria bassiana infection33. Furthermore, the intracellular Toll adaptor protein TmMyD88 has been implicated in the Toll signaling cascade34. Additionally, an early study from our team has characterized Cactin from T. molitor (TmCactin), a protein interacting with a Cactus homolog, and described its role as a positive regulator of the Toll pathway35. However, the events downstream of TmMyD88 have not yet been elucidated yet in T. molitor. The main purpose of this study was to analyze the developmental and tissue-specific expression level of TmDorX2 and to determine its mRNA expression patterns in response to bacterial and fungal insults. Moreover, we sought to clarify the effect of TmDorX2 silencing on AMP gene expression and survivability of T. molitor larvae in response to E. coli, S. aureus, and C. albicans challenge.
Materials and Methods
T. molitor rearing and culture of microbial strains
The mealworm T. molitor was reared on an artificial diet consisting of 170 g wheat flour, 0.5 g chloramphenicol, 20 g roasted soy flour, 0.5 g sorbic acid, 0.5 ml propionic acid, 10 g soy protein, and 100 g wheat bran, in 200 ml of distilled water which was autoclaved at 121 °C for 20 min. The insects were kept in an insectary at 27 ± 1 °C, and 60 ± 5% relative humidity (RH) in the dark. Healthy larvae at the 10th–12th instar (approximately 2.4 cm) were used for experiments. The Gram-negative bacteria Escherichia coli (strain K12), Gram-positive bacteria Staphylococcus aureus (strain RN4220), and the fungus Candida albicans were obtained from the American Type Culture Collection (ATCC) and used for immune challenge experiments. C. albicans suspension was prepared by culturing the fungi in Sabouraud Dextrose broth, whereas E. coli and S. aureus were cultivated in Luria-Bertani (LB) broth at 37 °C overnight. In order to use microorganisms for challenge experiments, overnight cultures were harvested, washed 3 times, and suspended in phosphate-buffered saline (PBS; pH 7.0). Subsequently, the cultures were centrifuged at 3,500 rpm for 15 min and OD600 values were measured using a spectrophotometer (Eppendorf, Germany). Based on the measured OD600 values, the microorganism cultures were adjusted to 106 cells/μl for E. coli and S. aureus, and 5 × 105 cells/μl for C. albicans.
Bioinformatics analysis for TmDorX2 identification and sequence characterization
To identify TmDorX2, a local-tblastn analysis was performed using the Tribolium castaneum Dorsal 2 (TcDorsal 2) amino acid sequence (GenBank: EFA02885.1) as the query, against the T. molitor nucleotide database. The deduced amino acid sequence was subjected to InterProScan 5.036 and blastx37 analyses for specific domain analysis predictions. The nuclear localization signal (NLS) was predicted by cNLS Mapper38. The selected amino acid sequences of each insect species, which include 5 beetles, 4 bees, 3 ants, 3 flies,3 mosquitos, 2 moths, 1 butterfly, and 1 stink bug (Table 1), were aligned using ClustalX239 to estimate the amino acid sequence similarity between TmDorX2 and its orthologs. The MEGA 7.0 program was utilized to create a phylogenetic tree40 using the maximum-likelihood (ML) method and the JTT matrix-based model41. The Dorsal protein of the Chinese white shrimp Penaeus chinensis (PcDorsal: ACJ36225.1) was used as an outgroup in the phylogenetic studies. A bootstrap analysis with 1000 replicates was used to derive the confidence of branches in the phylogenetic tree.
Table 1.
The accession number of Dorsal proteins used for bioinformatic analysis of this study.
| Name | Abbreviation | GenBank Number |
|---|---|---|
| Drosophila melanogaster dorsal, isoform D | DmDorD | NP_001163000.1 |
| Aedes albopictus embryonic polarity protein dorsal-like | AaDor | XP_019931750.1 |
| Anopheles glabripennis embryonic polarity protein dorsal-like isoform X3 | AgDor | XP_018572644.1 |
| Apis florea; embryonic polarity protein dorsal-like isoform X3 | AfDor | XP_012343274.1 |
| Apis mellifera dorsal protein isoform X2 | AmDor | XP_006567065.1 |
| Apis dorsata embryonic polarity protein dorsal-like isoform X5 | AdDorX5 | XP_006619742.1 |
| Bombus terrestris embryonic polarity protein dorsal isoform X2 | BtDor | XP_012174122.1 |
| Nicrophorus vespilloides putative transcription factor p65 homolog isoform X2 | NvDor | XP_017781152.1 |
| Asbolus verrucosus dorsal, partial | AvDor | RZB54393.1 |
| Tribolium castaneum Dorsal | TcDor | EFA02850.1 |
| Tribolium castaneum Dorsal 2 | TcDor2 | EFA02885.1 |
| Trachymyrmex cornetzi embryonic polarity protein dorsal isoform X3 | TcDorX3 | XP_018357420.1 |
| Halyomorpha halys embryonic polarity protein dorsal-like isoform X6 | HhDorX6 | XP_014275495.1 |
| Pogonomyrmex barbatus embryonic polarity protein dorsal isoform X3 | PbDorX3 | XP_006567063.1 |
| Monomorium pharaonis embryonic polarity protein dorsal isoform X2 | MpDorX2 | XP_011647341.1 |
| Delia antiqua Dorsal | DaDor | AFI98401.1 |
| Anopheles darlingi Rel1/Dif/Dorsal | AdDor | ETN66814.1 |
| Bombyx mori embryonic polarity protein dorsal isoform A | BmDorA | NP_001166296.1 |
| Spodoptera litura embryonic polarity protein dorsal isoform X4 | SlDorX4 | XP_022815079.1 |
| Pieris rapae embryonic polarity protein dorsal isoform X3 | PrDorX3 | XP_022116742.1 |
| Culex quinquefasciatus embryonic polarity protein dorsal | CqDor | XP_001844078.1 |
| Anopheles sinensis Dorsal isoform 1-B | AsDor1-B | KFB39849.1 |
| Penaeus chinensis dorsal | PcDor | ACJ36225.1 |
Gene expression analysis of TmDorX2 during different developmental stages and in various tissues
To elucidate the developmental pattern of TmDorX2 mRNA expression, samples were randomly collected from eggs (EG), young larvae (YL; 10th–12th instar), late instar larvae (LL; 19th–20th instar), pre-pupae (PP), 1 to 7-day-old pupae (P1-P7), and 1- to 5-day-old adults (n = 20 for each stage). To elucidate the tissue-specific pattern of TmDorX2 mRNA expression, larval and adult tissues of T. molitor including the fat body, Malpighian tubules (MTs), gut, integument, hemocytes, ovary, and testis, were dissected. Subsequently, total RNA was extracted from the collected samples following the LogSpin RNA isolation method with minor modifications42. Briefly, the tissue samples were homogenized in guanidine thiocyanate based RNA lysis buffer (20 mM EDTA, 20 mM MES buffer, 3 M guanidine thiocyanate, 200 mM sodium chloride, 40 μM phenol red, 0.05% Tween-80, 0.5% glacial acetic acid (pH 5.5), and 1% isoamyl alcohol in 50 ml) using a bead-based homogenizer (Bertin Technologies, France). After incubation at room temperature (approximately 25 °C) for 1 min, the samples were centrifuged at 15,000 rpm for 5 min at 4 °C. Subsequently, 100 μl from the supernatants were diluted in 200 μl of RNA lysis buffer added to 300 μl of 99.9% ethanol. and were centrifuged at 15,000 rpm for 30 s at 4 °C, using silica spin columns (Bioneer, Korea, KA-0133-1). The aqueous phase was discarded, and the genomic DNA was digested using DNase (Promega, USA, M6101) for 15 min at 37 °C. Subsequently, the silica spin columns were washed with 450 μl of 3 M sodium acetate buffer by centrifugation at 15,000 rpm for 30 s at 4 °C. Next, 500 μl of 80% ethanol was added to the spin columns and the samples were centrifuged again. After drying the spin column for 1 min, total RNA was eluted in 30 µl of distilled water for cDNA synthesis and other downstream applications. 2 μg of total RNAs were used for cDNA synthesis by using the AccuPower® RT PreMix (Bioneer, Korea) kit with an oligo-(dT)12–18 primer according to the manufacturer’s instructions.
The synthesized cDNAs (1:20 dilution with DNase/RNase free water) were used as template for quantitative reverse-transcription PCR (qRT-PCR). The qRT-PCR analyses of cDNA samples were performed in AccuPower® 2X GreenStar qPCR Master Mix (Bioneer), containing the specifically designed primers, TmDorX2-qPCR-Fw and TmDorX2-qPCR-Rv (Table 2) (Fig. S1). Primers for TmDorX2 and T. molitor ribosomal protein (TmL27a), which was used as an internal control were designed using Primer 3.0 plus (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi). The PCR amplification conditions were as follows: 95 °C for 5 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 30 s. Finally, TmDorX2 gene expression was evaluated using the comparative CT method (2−ΔΔCT method)43.
Table 2.
Sequences of the primers used in this study.
| Name | Primer sequences |
|---|---|
| TmDorX2_qPCR_Fw | 5′-ACACCCCCGAAATCACAAAC-3′ |
| TmDorX2_qPCR_Rv | 5′-TTTCAGAGCGCCAGGTTTTG-3′ |
| TmDorX2_Temp_Fw | 5′- AAACCTGGCGCTCTGAAAC-3′ |
| TmDorX2_Temp_Rv | 5′-CAGGTGAATCGGTTGGAGTT-3′ |
| dsTmDorX2_Fw | 5′-TAATACGACTCACTATAGGGT CTATCTAGCTGGCAGGGACG-3′ |
| dsTmDorX2_Rv | 5′-TAATACGACTCACTATAGGGT CAGGTGAATCGGTTGGAGTT-3′ |
| dsEGFP_Fw | 5′-TAATACGACTCACTATAGGGT ACGTAAACGGCCACAAGTTC-3′ |
| dsEGFP_Rv | 5′-TAATACGACTCACTATAGGGT TGCTCAGGTAGTGTTGTCG-3′ |
| TmTenecin-1_Fw | 5′-CAGCTGAAGAAATCGAACAAGG-3′ |
| TmTenecin-1_Rv | 5′-CAGACCCTCTTTCCGTTACAGT-3′ |
| TmTenecin-2_Fw | 5′-CAGCAAAACGGAGGATGGTC-3′ |
| TmTenecin-2_Rv | 5′-CGTTGAAATCGTGATCTTGTCC-3′ |
| TmTenecin-3_Fw | 5′-GATTTGCTTGATTCTGGTGGTC-3′ |
| TmTenecin-3_Rv | 5′-CTGATGGCCTCCTAAATGTCC-3′ |
| TmTenecin-4_Fw | 5′-GGACATTGAAGATCCAGGAAAG-3′ |
| TmTenecin-4_Rv | 5′-CGGTGTTCCTTATGTAGAGCTG-3′ |
| TmDefensin-1_Fw | 5′-AAATCGAACAAGGCCAACAC-3′ |
| TmDefencin-1_Rv | 5′-GCAAATGCAGACCCTCTTTC-3′ |
| TmDefencin-2_Fw | 5′-GGGATGCCTCATGAAGATGTAG-3′ |
| TmDefencin-2_Rv | 5′-CCAATGCAAACACATTCGTC-3′ |
| TmColeoptericin-1_Fw | 5′-GGACAGAATGGTGGATGGTC-3′ |
| TmColeoptericin-1_Rv | 5′-CTCCAACATTCCAGGTAGGC-3 |
| TmColeoptericin-2_Fw | 5′-GGACGGTTCTGATCTTCTTGAT-3′ |
| TmColeoptericin-2_Rv | 5′-CAGCTGTTTGTTTGTTCTCGTC-3′ |
| TmAttacin-1a_Fw | 5′-GAAACGAAATGGAAGGTGGA-3′ |
| TmAttacin-1a_Rv | 5′-TGCTTCGGCAGACAATACAG-3′ |
| TmAttacin-1b_Fw | 5′-GAGCTGTGAATGCAGGACAA-3′ |
| TmAttacin-1b_Rv | 5′-CCCTCTGATGAAACCTCCAA-3′ |
| TmAttacin-2_Fw | 5′-AACTGGGATATTCGCACGTC-3′ |
| TmAttacin-2_Rv | 5′-CCCTCCGAAATGTCTGTTGT-3 |
| TmCecropin-2_Fw | 5′-TACTAGCAGCGCCAAAACCT-3′ |
| TmCecropin-2_Rv | 5′-CTGGAACATTAGGCGGAGAA-3′ |
| TmThaumatin-like protein-1_Fw | 5′-CTCAAAGGACACGCAGGACT-3′ |
| TmThaumatin-like protein-1_Rv | 5′-ACTTTGAGCTTCTCGGGACA-3′ |
| TmThaumatin-like protein-2_Fw | 5′-CCGTCTGGCTAGGAGTTCTG-3′ |
| TmThaumatin-like protein-2_Rv | 5′-ACTCCTCCAGCTCCGTTACA-3′ |
| TmL27a_qPCR_Fw | 5′-TCATCCTGAAGGCAAAGCTCCAGT-3′ |
| TmL27a_qPCR_Rv | 5′-AGGTTGGTTAGGCAGGCACCTTTA-3′ |
※Underline indicates T7 promoter sequences.
Analysis of TmDorX2 mRNA expression after microbial challenge
In order to determine TmDorX2 mRNA expression upon microbial challenge, T. molitor larvae (10th–12th instar larvae) were experimentally challenged by injecting 1 µl of E. coli (1 × 106 cells/µl), S. aureus (1 × 106 cells/µl), and/or C. albicans (5 × 104 cells/µl) into separate groups of larvae. Immune tissues, including the fat body, hemocytes, gut, and MTs, were collected from each of the microbe-infected group and from PBS-injected groups acting as a wounding control at 3, 6, 9, 12, and 24 h post-infection. Subsequently, total RNA extraction, cDNA synthesis, and qRT-PCR were carried out as described above.
TmDorX2 gene silencing
To prepare double-stranded RNA against TmDorX2 (dsTmDorX2), we designed forward and reverse primers containing the T7 promoter sequence at their 5′ ends using the SnapDragon-Long dsRNA design software (https://www.flyrnai.org/cgi-bin/RNAi_find_primers.pl) (Table 2) (Fig. S1). The 480-bp PCR product was amplified in AccuPower® Pfu PCR PreMix with the TmDorX2_Temp_Fw and TmDorX2_Temp_Rv (Table 2) (Fig. S1) at 95 °C for 2 min, followed by 30 cycles of denaturation at 95 °C for 20 s, annealing at 56 °C for 30 s, and extension at 72 °C for 5 min. The procedure was followed using the same PCR conditions, which led to production of a 388-bp PCR product containing the T7 promoter sequence (Table 2). The synthesized dsTmDorX2 was purified, using an AccuPrep® PCR Purification Kit (Bioneer, Korea), precipitated with 5 M ammonium acetate, and washed with 70% ethanol. Subsequently, the PCR product was used as a template to synthesize dsTmDorX2 in vitro using an EZTM T7 High Yield in vitro Transcription Kit (Enzynomics, Korea) as per the manufacturer’s instructions. Briefly, 1 μg of the final PCR product was mixed with 4 μl of 5X Transcription Buffer, 2 μl of 10X MgCl2, 2 μl of DTT (100 mM), 1 μl of RNase Inhibitor (40 U/μl), 1 μl of rATP (100 mM), 1 μl of rGTP (100 mM), 1 μl of rCTP (100 mM), 1 μl of rUTP (100 mM), and 1 μl of T7 RNA polymerase. Subsequently, the mixture was incubated at 37 °C for 3 h and 25 °C for 1 h. The synthesized dsTmDorX2 was mixed with one volume of 5 M ammonium acetate, incubated on ice for 15 min, and washed three times using 70%, 80% and 99.9% ethanol, respectively. Finally, after drying, the pellet was resuspended in 30 μl distilled water (Sigma, USA, W4502-1L). A 546 bp PCR product of the Enhanced Green Fluorescent Protein (EGFP) gene (derived from the plasmid EGFP-C1) was used as a template to synthesize double-stranded EGFP (dsEGFP) acting as negative control.
Then, to address the importance of TmDorX2 in T. molitor humoral immunity, 1 μl (1 μg) of dsTmDorX2 was injected into T. molitor 10th–12th instar larvae. Control insects were injected with dsEGFP. The efficacy of dsTmDorX2 gene silencing was confirmed by qRT-PCR at 2 days post-injection. After confirmation of silencing, dsRNA-injected larvae (n = 10 per group) were infected with E. coli (1 × 106 cells/larva), S. aureus (1 × 106 cells/larva), or C. albicans (5 × 104 cells/larva), and mortality was monitored every day for a duration of 10 days. The experiment was repeated three times with 10 larvae per group for each experiment.
Analysis of TmDorX2 silencing on AMP expression post-microbial challenge
To elucidate the function of the protein encoded by the TmDorX2 transcript in regulating AMP genes, the gene expression profile of 14AMPs including TmTenecin-1 (TmTene1), TmTenecin-2 (TmTene2), TmTenecin-3 (TmTene3), TmTenecin-4 (TmTene4), TmAttacin-1a (TmAtt1a), TmAttacin-1b (TmAtt1b), TmAttacin-2 (TmAtt2), TmDefensin-1 (TmDef1), TmDefensin-2 (TmDef2), TmColeoptericin-1 (TmCole1), TmColeoptericin-2 (TmCole2), TmCecropin-2 (TmCec2), TmThaumatin-like protein-1 (TmTLP1), and TmThaumatin-like protein-2 (TmTLP2) were determined in the TmDorX2-silenced larvae after microbial challenge. dsEGFP was used as the negative control, and PBS served as the wound control. Experimental samples were dissected 24 h post-injection and the following immune-sensing tissues were isolated: the fat body, hemocytes, gut, and MTs. The samples were processed for cDNA synthesis, and qRT-PCR analysis was conducted using AMP-specific primers (Table 2).
Statistical analysis
All experiments were carried out in triplicate and data were subjected to one-way analysis of variance (ANOVA). In order to evaluate the difference between groups (p < 0.05), the Tukey’s multiple range test was performed. The results for the mortality assay were analyzed using the Kaplan-Meier plot (log-rank Chi-square test) in Excel (http://www.real-statistics.com/survival-analysis/kaplan-meier-procedure/real-statistics-kaplan-meier/).
Results
Gene organization, cDNA analysis, and phylogenetic tree
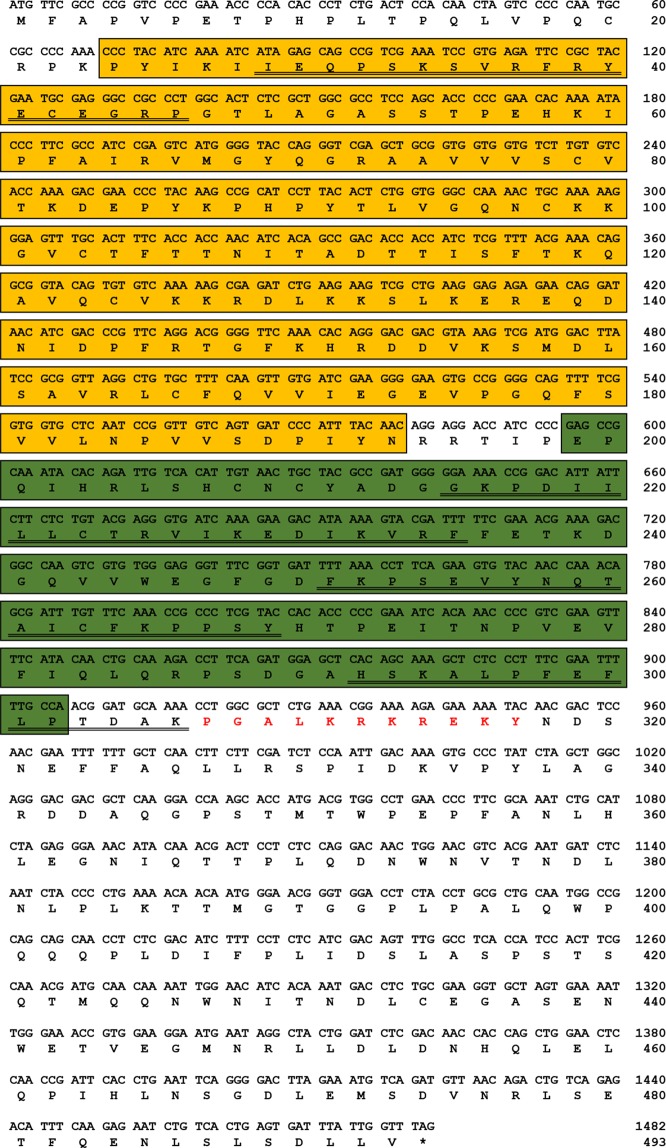
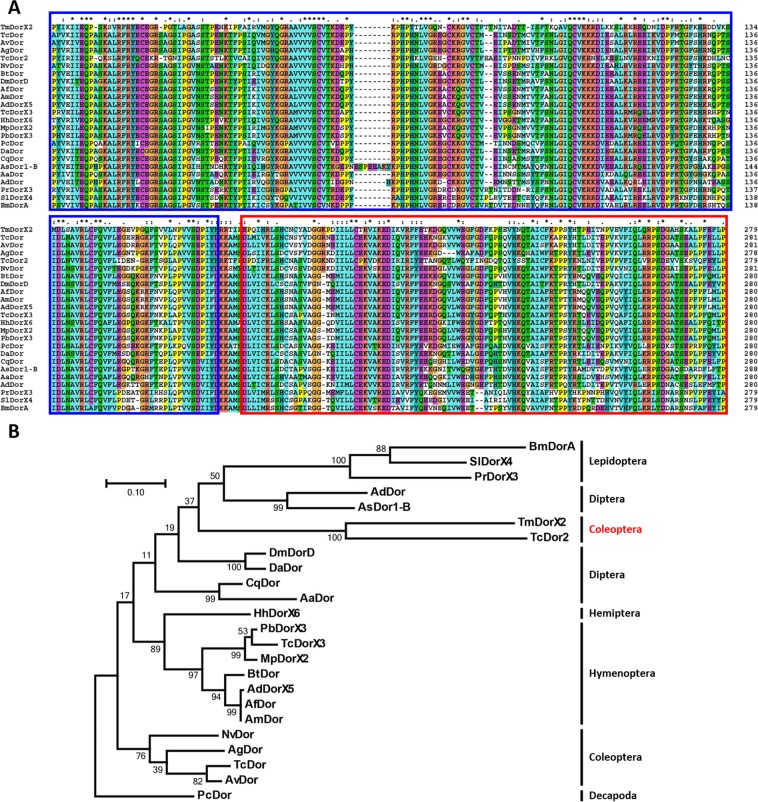
A local tblastn search using the T. castaneum Dorsal 2 protein sequence (GenBank: EFA02885.1) as the query and the T. molitor RNAseq and EST libraries as the subject was sufficient to identify the Dorsal homologue from T. molitor (Designated as TmDorX2; accession number: MN056348). The genomic organization of TmDorX2 showed that it contains five exons and four introns (Fig. S2). The TmDorX2 full-length ORF consists of 1,482 bp, encoding a polypeptide of 493 amino acid residues (Fig. 1). According to InterProScan analysis and the NCBI conserved domain database, the TmDorX2 amino acid sequence comprises a RHD (P24 to N193), an IPT (E199 to P302), and a nuclear localization signal (NLS; P307GALKRKREKY317). Four putative NF-κB signature sequences (I28 to P46; G215 to F235; F251 to Y269; H292 to K306) were found at the N-terminus of the RHD and the N- and C-terminusof the IPT domain. To evaluate the evolutionary position of TmDorX2 using percentage identity and phylogenetic analysis, we retrieved orthologous sequences from 22 insect species. Furthermore, the conserved RHD and IPT domain in TmDorX2 were compared at the amino acid level using ClustalX 2.1 multiple sequence alignment (Fig. 2A).
Figure 1.
The full-length ORF sequence of the T. molitor Dorsal isoform X2 (TmDorX2) and its deduced amino acid sequence. Nucleotide and amino acid numbers are shown at the right margin, showing that the TmDorX2 ORF sequence contains 1,482 bp nucleotides encoding 493 amino acid residues. The sequence at the N-terminal region is representing the Rel homology domain (RHD) is highlighted in the yellow box, and that of the immunoglobulin-like, plexins, transcription factors (IPT) domain is highlighted in the green box. The nuclear localization signal (NLS; P307GALKRKREKY317) is shown in red. The transcription factor NF-κB signature sequences are indicated with a double underline.
Figure 2.
Multiple sequence alignment of TmDorX2 with its orthologues (A) and phylogenetic analysis (B). The sequences of representative Dorsal proteins showed high homology at the conserved Rel homology domains (RHD) marked in blue boxes and the immunoglobulin-like, plexins, transcription factors (IPT) domains marked in red boxes. The symbols indicate conservation scores between groups according to the Gonnet PAM 250 matrix (‘*’>‘:’>‘.’) and ‘−’ indicates internal or terminal gaps (A). The phylogenetic tree was constructed using the maximum likelihood method and bootstrapped 1,000 times in the MEGA 7.0 program based on the multiple alignment by ClustalX2.1. Protein sequences used in this study are as follows: TmDorX2 (Tenebrio molitor Dorsal protein isoform X2), DmDorD (Drosophila melanogaster Dorsal, isoform D; NP_001163000.1), AaDor (Aedes albopictus embryonic polarity protein dorsal-like; XP_019931750.1), AgDor; (Anopheles glabripennis embryonic polarity protein dorsal-like isoform X3; XP_018572644.1), AfDor (Apis florea; embryonic polarity protein dorsal-like isoform X3; XP_012343274.1), AmDor (Apis mellifera dorsal protein isoform X2; XP_006567065.1), AdDorX5 (Apis dorsata embryonic polarity protein dorsal-like isoform X5; XP_006619742.1), BtDor (Bombus terrestris embryonic polarity protein dorsal isoform X2; XP_012174122.1), NvDor (Nicrophorus vespilloides putative transcription factor p65 homolog isoform X2; XP_017781152.1), AvDor (Asbolus verrucosus dorsal, partial; RZB54393.1), TcDor (Tribolium castaneum Dorsal; EFA02850.1), TcDor2 (Tribolium castaneum Dorsal 2; EFA02885.1), TcDorX3 (Trachymyrmex cornetzi embryonic polarity protein dorsal isoform X3; XP_018357420.1), HhDorX6 (Halyomorpha halys embryonic polarity protein dorsal-like isoform X6; XP_014275495.1), PbDorX3 (Pogonomyrmex barbatus embryonic polarity protein dorsal isoform X3; XP_006567063.1), MpDorX2 (Monomorium pharaonis embryonic polarity protein dorsal isoform X2; XP_011647341.1), DaDor (Delia antiqua Dorsal; AFI98401.1), AdDor (Anopheles darlingi Rel1/Dif/Dorsal; ETN66814.1), BmDorA (Bombyx mori embryonic polarity protein dorsal isoform A; NP_001166296.1), SlDorX4 (Spodoptera litura embryonic polarity protein dorsal isoform X4; XP_022815079.1), PrDorX3 (Pieris rapae embryonic polarity protein dorsal isoform X3; XP_022116742.1), CqDor (Culex quinquefasciatus embryonic polarity protein dorsal; XP_001844078.1), and AsDor1-B (Anopheles sinensis Dorsal isoform 1-B; KFB39849.1). PcDor (Penaeus chinensis dorsal; ACJ36225.1) was used as an outgroup (B).
Phylogenetic analysis revealed that the Dorsal isoforms from representative insect species clustered under separate insect orders (Fig. 2B). The Dorsal X2 isoforms from T. molitor and T. castaneum were clustered together, as confirmed by bootstrap replications. The Dorsal isoforms from the order Coleoptera were clustered separately. Similarly, the Dorsal isoforms from insect orders Lepidoptera, Hemiptera and Hymenoptera were classified into separate independent clusters. Moreover, species belonging to the order Hymenoptera (including ants and bees) formed two distinct clusters, one formed by ants [PbDorX3 (Pogonomyrmex barbatus), TcDorX3 (Trachymyrmex cornetzi), and MpDorX2 (Monomorium pharaonis)] and another by bees [BtDor (Bombus terrestris), AdDorX5 (Apis dorsata), AfDor (Apis florea), and AmDor (A. mellifera)]. Whereas Drosophila Dorsal isoforms showed relatedness, the Dorsal isoforms belonging to other species in the order Diptera were clustered separately.
Percent identity calculated based on specific domain analysis showed that TmDorX2 has the highest similarity with TcDor and AvDor (64% identity), followed by 59% and 57% identity with TcDor2 and NvDor, respectively. In addition, the maximum and minimum identities of TmDorX2 within the Hymenoptera (56–57%), Diptera (51–54%), and Lepidoptera (46–49%) orders were calculated and are presented in Fig. S3.
Temporal and spatial expression patterns of TmDorX2
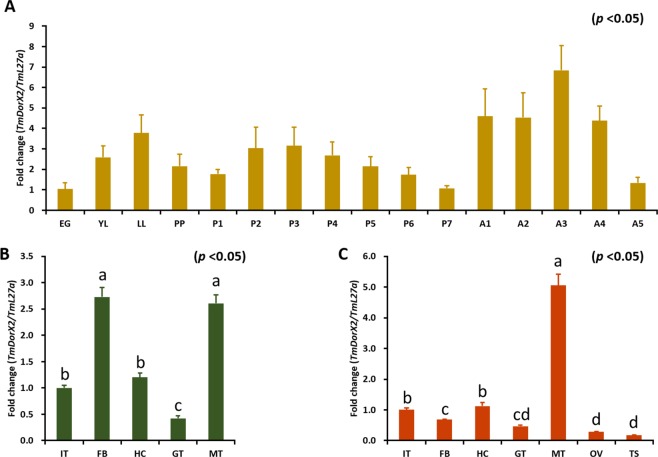
qRT-PCR was employed to investigate TmDorX2 mRNA expression during different developmental stages of the insect relative to the levels of TmL27a as an internal control (Fig. 3A). The TmDorX2 mRNA was expressed during all developmental stages of T. molitor. The mRNA levels were upregulated in the late larval stage, followed by a decline in expression in early pupae. This was followed by an increase in mRNA expression in 2-day old pupae and a consistent decline in late pupal stages. The expression of TmDorX2 was higher during adult stages, with the highest expression observed in 3-day-old adults (Fig. 3A). The TmDorX2 mRNA was detected in all T. molitor larval tissues with the highest expression level observed in the fat body and MTs, followed by that in the integuments and hemocytes, while the lowest expression was observed in the gut (Fig. 3B). Likewise, the transcription of TmDorX2 in 5-old-day adults was significantly higher in MTs, followed by that in the integument and hemocytes. The lowest expression of TmDorX2 was found in reproductive tissues (Fig. 3C).
Figure 3.
Relative TmDorX2 mRNA levels during development (A) and in different tissues of T. molitor late-instar larvae (B) and 5-day-old adults (C) measured by qRT-PCR. (A) TmDorX2 expression levels are shown for eggs (EG), young larvae (YL), late-instar larvae (LL), Pre-pupa (PP), 1 to 7-day-old pupa (P1-P7), and 1 to 5-day-old adults (A1-A5). (B) The expression levels of TmDorX2 in the integument (IT), fat body (FB), hemocytes (HC), gut (GT), and Malpighian tubules (MT) are shown for late-instar larvae. In addition to the above tissues, the expression levels of TmDorX2 in the ovary (OV) and testis (TS) is depicted in 5-day-old adults (C). The Y-axis represents the relative expression level of TmDorX2. The T. molitor 60 S ribosomal protein L27a (TmL27a) served as an endogenous control to normalize RNA levels between samples. Vertical bars indicate mean ± SE (n = 20).
Induction profile of TmDorX2 upon microbial insult
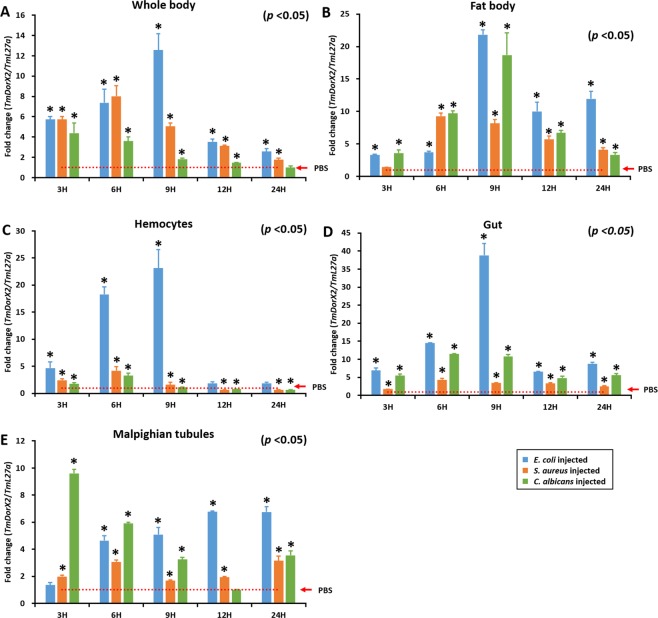
To elucidate the participation of TmDorX2 in T. molitor innate immunity upon microbial infection, we evaluated the levels of TmDorX2 mRNA expression by qRT-PCR at different time points (3, 6, 9, 12, and 24 h) in the fat body, hemocytes, gut, MTs, and the whole body of T. molitor larvae (Fig. 4). In Tenebrio larvae injected with Gram-negative bacteria E. coli, the whole-body levels of TmDorX2 expression increased 9 h post-injection (hpi), followed by a gradual decline at 12 hpi and 48 hpi (Fig. 4A). Significant increase in whole-body TmDorX2 expression was also observed after S. aureus infection, at all time points tested, when compared with that in the mock control (p < 0.05). In the fat body tissue, the fold-increase in TmDorX2 expression upon infection with E. coli and the fungus C. albicans reached its highest level at 9 hpi. S. aureus infection also caused a significant increase in TmDorX2 expression in the fat body when compared with that in the mock-infected larvae (p < 0.05) (Fig. 4B). In hemocytes, E. coli infection led to a dramatic increase in TmDorX2 expression at early stages of infection followed by a large decline at later time points (Fig. 4C). Similar TmDorX2 expression profiles were observed in the gut after E. coli infection. The increase in TmDorX2 expression in the gut was significant when compared with the mock control (p < 0.05) (Fig. 4D). In the MTs, an early increase in the expression of TmDorX2 mRNA was observed after C. albicans infection, which was statistically significant (p < 0.05) (Fig. 4E).
Figure 4.
Temporal expression profiles of TmDorX2 in response to E. coli, S. aureus, and C. albicans infection in the whole body (A), fat body (B), hemocytes (C), gut (D), and Malpighian tubules (E) of T. molitor young larvae (10th to 12th instar larvae), examined by qRT-PCR at 3, 6, 9, 12, at 24 h post-infection. TmDorX2 expression levels were normalized to their levels in phosphate buffered saline (PBS)-injected controls. The T. molitor 60 S ribosomal protein L27a (TmL27a) was used as an internal control. Vertical bars indicate mean ± SE (n = 20). ‘*’ Indicates significant differences (p < 0.05).
Mortality upon TmDorX2 knockdown and microbial challenge
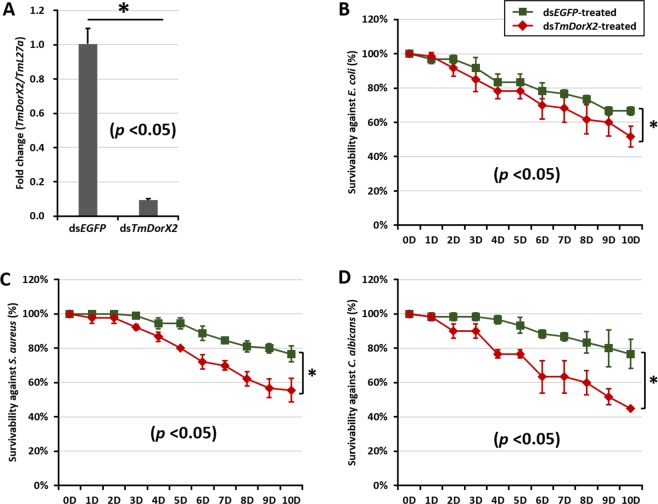
Double-stranded RNA (dsRNA) against TmDorX2 was synthesized and used to assess the involvement of TmDorX2 in the Toll pathway. Subsequently, the transcription of 14 AMP genes was examined to determine their regulation by the Toll signaling pathway. Evaluation of the levels of TmDorX2 mRNA expression after dsRNA injection in comparison to those in the dsEGFP treated negative control demonstrated a 91% knockdown efficiency for TmDorX2. (Fig. 5A). To examine the effect of TmDorX2 silencing on larval survival after challenging with E. coli, S. aureus, and C. albicans, we performed experiments in two sets of T. molitor larvae treated with dsTmDorX2 and dsEGFP, respectively, and counted the dead insects for 10 days. Larval mortality was compared between the dsEGFP- and dsTmDorX2-treated groups and a difference at p < 0.05 was considered significant. The results show that the mortality rate resulting from E. coli (48%, Fig. 5B) and S. aureus (44%, Fig. 5C) infections in the dsTmDorX2-treated groups were significantly different from that in the corresponding dsEGFP groups, respectively. The percent mortality of C. albicans challenged larvae was 55%, and was significantly different than that of the dsEGFP-treated larvae (p < 0.05) (Fig. 5D).
Figure 5.
Effect of TmDorX2 dsRNA (RNAi) on the survival of T. molitor larvae (n = 10 per group), observed for 10 days after microbial insult. (A) Silencing efficiency of TmDorX2 RNAi assessed by measuring the TmDorX2 mRNA levels by qRT-PCR 2 days post-injection. Survival of T. molitor larvae injected with dsTmDorX2 after immune challenge with (B) E. coli (C) S. aureus, and (D) C. albicans. dsEGFP injected groups acted as negative control. The data are an average of three independent biological replicate experiments. ‘*’ Represents significant differences between dsTmDorX2- and dsEGFP-treated groups (p < 0.05).
Role of TmDorX2 in T. molitor AMP gene expression
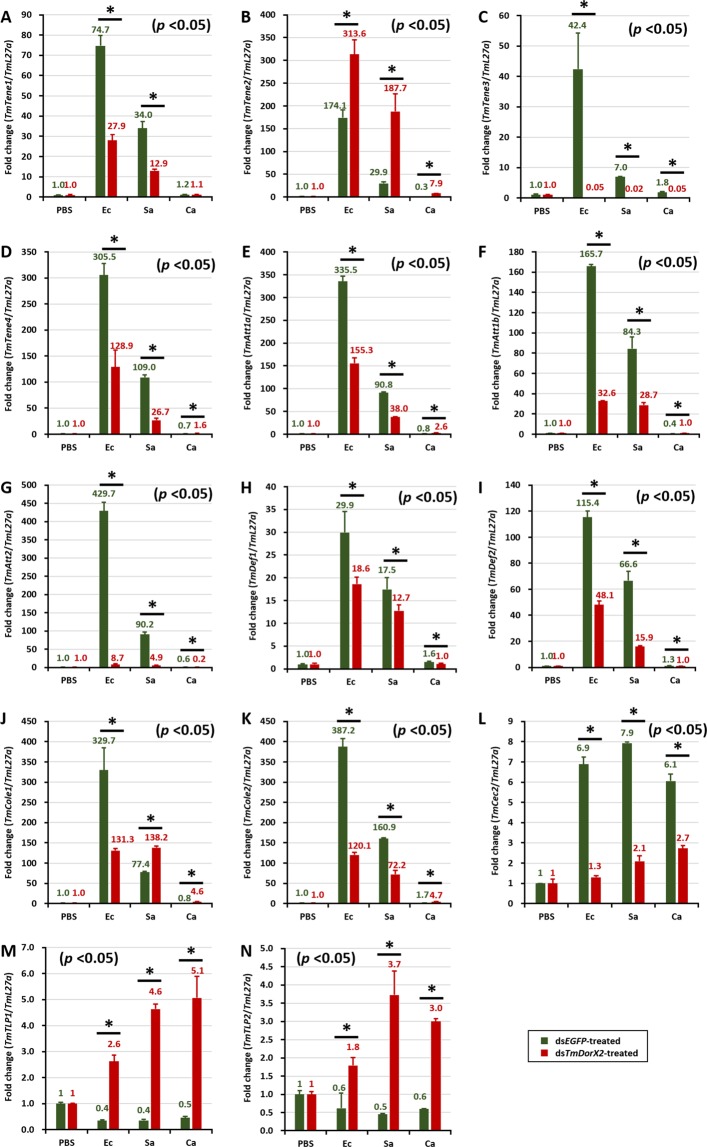
Given that the stimulation of immune signaling cascades leads to the production of antibacterial or antifungal AMPs to combat the invading pathogens, we investigated the expression of 14 T. molitor AMP genes, namely TmTene1, −2, −3, and −4, TmAtt1a, −1b, and −2, TmDef1 and −2, TmCole1 and −2, TmCec2, and TmTLP1 and −2. We hypothesized that the significant mortality observed in the dsTmDorX2-treated group after microbial challenge was due to the downregulation of AMP gene expression. To confirm our hypothesis, experiments were performed with two groups of T. molitor larvae, injected with 1 μl (1 μg) of TmDorX2 dsRNA and dsEGFP, respectively. After confirming TmDorX2 silencing 2 days after dsRNA injection, larvae were injected with E. coli, S. aureus, C. albicans, or PBS. One-day post-microbial infection, the AMP gene expression profile was studied by qRT-PCR in dissected immune tissues, such as the fat body, hemocytes, gut, and MTs.
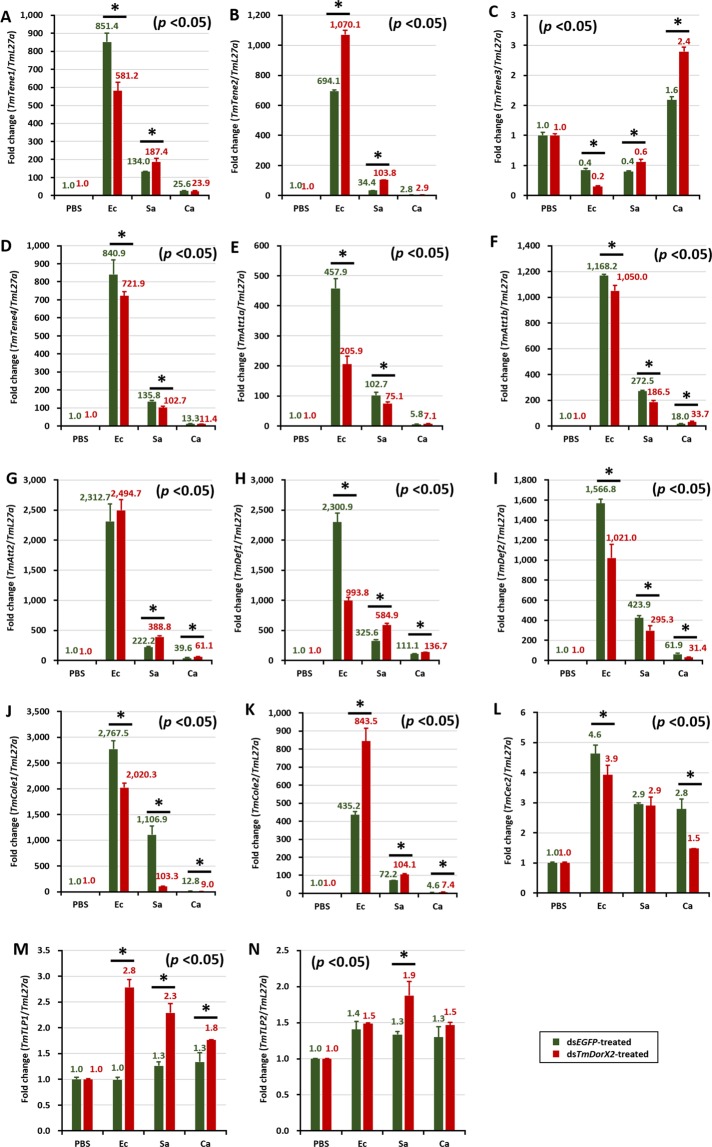
In the fat body of TmDorX2-silenced larvae, the expression of 11 AMP genes namely TmTene1, −3 and −4 (Fig. 6A,C,D), TmAtt1a, −1b and −2 (Fig. 6E–G), TmDef1 and −2 (Fig. 6H,I), TmCole1, and −2 (Fig. 6J,K), and TmCec2 (Fig. 6L), was noticeably decreased upon E. coli challenge (Fig. 6). In contrast, the transcription of TmTene2 (Fig. 6B), TmTLP1 (Fig. 6M), and TmTLP2 (Fig. 6N) was upregulated in dsTmDorX2-injected larvae upon E. coli, S. aureus, or C. albicans challenge, as compared to that in dsEGFP injected larvae. Simultaneously, there was a considerable decline in the expression of the same 11 AMP genes, as well as in the TmTene2 mRNA level, in the gut (Fig. 7). Furthermore, TmDorX2 knockdown reduced the expression of 8 AMPs in hemocytes and MTs of E. coli challenged larvae. This suggests that the downregulation of AMP gene expression in the fat body and gut of dsTmDorX2 larvae after E. coli infection is more striking than the reduction of AMP genes in hemocytes and MTs. Upon S. aureus infection, the transcription of 10 AMPs, namely TmTene1, −3, and −4 (Fig. 6A,C,D), TmAtt1a, −1b and −2 (Fig. 6E–G), TmDef1 and −2 (Fig. 6H,I), TmCole2 (Fig. 6K), and TmCec2 (Fig. 6L), was upregulated in the fat body of dsEGFP-treated groups, whereas it was decreased in dsTmDorX2-injected groups, suggesting that the fat body is a crucial tissue for combatting S. aureus infection. In addition to changes in the fat body, S. aureus infection caused a significant downregulation of AMPs TmTene1 (Fig. 7A), TmAtt1a, 1b, and 2 (Fig. 7E–G), TmDef1 and 2 (Fig. 7H,I), TmCole1 and 2 (Fig. 7J,K) in hemocytes as well.
Figure 6.
Antimicrobial peptide (AMP) induction patterns in TmDorX2-silenced T. molitor larval fat body in response to E. coli (Ec), S. aureus (Sa), or C. albicans (Ca) infection. Gram-negative bacteria (E. coli), Gram-positive bacteria (S. aureus), and fungi (C. albicans) were injected into dsTmDorX2-treated T. molitor larvae on the second day post-dsRNA injection, the larvae were dissected 24 h post-microbial infection. The expression levels of the following AMP genes were analyzed by qRT-PCR: TmTenecin-1 (TmTene1, A); TmTenecin-2 (TmTene2, B); TmTenecin-3 (TmTene3, C); TmTenecin-4 (TmTene4, D); TmAttacin-1a (TmAtt1a, E); TmAttacin-1b (TmAtt1b, F); TmAttacin-2 (TmAtt2, G); TmDefensin-1 (TmDef1, H); TmDefensin-2 (TmDef2, I);TmColeptericin-1 (TmCole1, J); TmColeptericin-2 (TmCole2, K); TmCecropin-2 (TmCec2, L); TmTLP-1 (TmTLP1, M); and TmTLP-2 (TmTLP2, N) dsEGFP was used as a negative control and TmL27a served as an internal control. The numbers above the bars represent the AMP transcription levels. All experiments were repeated three times with similar results.
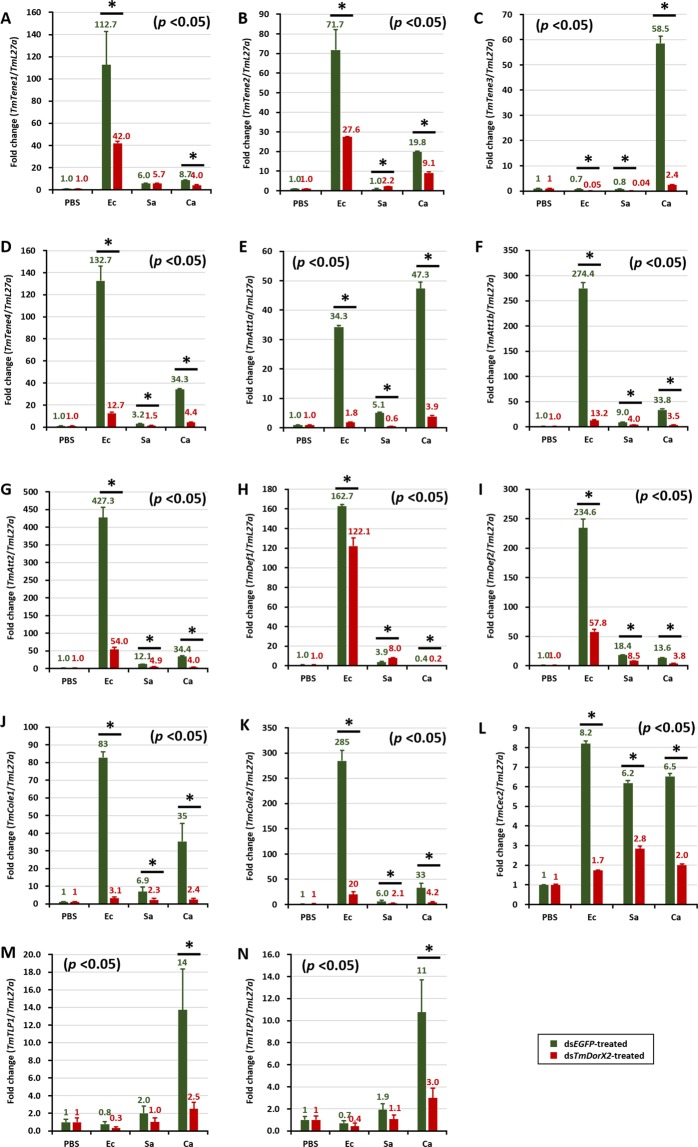
Figure 7.
Effect of dsTmDorX2 on antimicrobial peptide (AMP) gene expression in T. molitor larval gut infected with E. coli (Ec), S. aureus (Sa), and C. albicans (Ca). On the second day after dsRNA TmDorX2 injection, at which point the mRNA levels of TmDorX2 were reduced by 91% in the dsTmDorX2-treated groups compared to the dsEGFP-treated groups, the larvae were exposed to the microbes. The expression levels of 14 AMP genes were analyzed by qRT-PCR: TmTene1 (A); TmTene2 (B); TmTene3 (C); TmTene4 (D); TmAtt1a (E); TmAtt1b (F); TmAtt2 (G); TmDef1 (H); TmDef2 (I); TmCole1 (J); TmCole2 (K); TmCec2 (L); TmTLP-1 (M); and TmTLP-2 (N). dsEGFP was used as a negative control and TmL27a served as an internal control. The numbers above the bars show the transcript expression levels. Statistical analysis was performed using Student’s t-tests (p < 0.05).
Intriguingly, the expression of TmTene2, −3 and −4 (Fig. 7B–D), TmAtt1a, −1b, and −2 (Fig. 7E–G), TmCole1 and −2 (Fig. 7J,K), TmCec2 (Fig. 7L), and TmTLP1 and −2 (Fig. 8M,N) in the gut of dsEGFP-treated groups were upregulated by C. albicans and bacterial infection, whereas it declined markedly in dsTmDorX2-treated larvae. In addition, levels of the antifungal AMPs TmTene3, TmTLP1 and TmTLP2 (Fig. 8C,M,N) were noticeably higher in dsEGFP groups and lower in the dsTmDorX2-treated groups upon C. albicans infection, suggesting that TmDorX2 specifically regulates antifungal AMPs in the gut in response to C. albicans.
Figure 8.
Expression levels of antimicrobial peptides (AMPs) in TmDorX2-silenced T. molitor hemocytes upon E. coli, S. aureus, or C. albicans infection. The qRT-PCR expression profiles of TmTene1 (A), TmTene-2 (B), TmTene3 (C), TmTene4 (D), TmDef1 (E), TmDef2 (F), TmCec2 (G), TmCole1 (H), TmCole2 (I), TmAtt1a (J), TmAtt1b (K), TmAtt2 (L), TmTLP1 (M), and TmTLP2 (N) are shown. dsEGFP was used as a negative control and TmL27a was used as an internal control. All experiments were performed at least three times, and statistical analysis was performed using Student’s t-tests (p < 0.05).
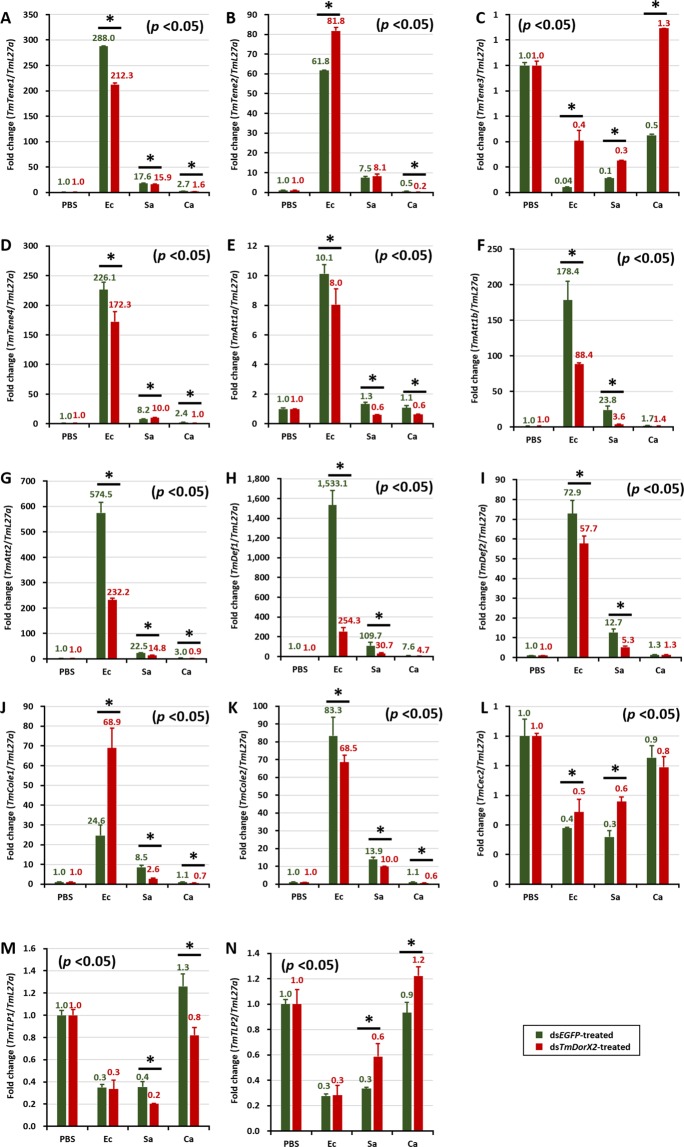
In MTs, the requirement for TmDorX2 against C. albicans challenge was less marked as compared to that in the gut tissue. As shown in Figs 9 and 10, AMPs, such as TmTene1, TmTene4, TmAtt1a, TmDef1, TmDef2 and TmCole1, were downregulated in dsTmDorX2- injected larvae challenged with E. coli, S. aureus, and C. albicans. Moreover, TmTene4 (Fig. 9D), TmAtt1a, 1b (Fig. 9E,F), TmDef2 (Fig. 9I), and TmCole1 (Fig. 9J) showed decreased expression in TmDorX2-depleted groups compared to controls upon S. aureus challenge, similar to other tissues. However, S. aureus induced the expression of TmTene1, TmTene2 (Fig. 9A,B), TmAtt2 (Fig. 9G), TmDef1 (Fig. 9H), TmCole2 (Fig. 9K), TmTLP1, and TmTLP2 (Fig. 9M,N) in dsTmDorX2-treated larvae. During C. albicans, similar to S. aureus, the mRNA levels of TmAtt2 (Fig. 9G), TmDef1 (Fig. 9H), TmCole2 (Fig. 9K), TmTLP1, and TmTLP2 (Fig. 9M,N) were increased in the TmDorX2-silenced groups, whereas the expression of TmDef2 (Fig. 9I) and TmCole1 (Fig. 9J) was decreased.
Figure 9.
Antimicrobial peptide (AMP) expression levels in TmDorX2-knockdown T. molitor larval MTs upon E. coli (Ec), S. aureus (Sa), or C. albicans (Ca) infection on the second day post-TmDorX2 silencing. Individuals from each group were dissected 24 h after microbial challenge. The expression levels of the following AMPs were measured by qRT-PCR:TmTenecin-1 (TmTene1, A); TmTenecin-2 (TmTene2, B); TmTenecin-3 (TmTene3, C); TmTenecin-4 (TmTene4, D); TmAttacin-1a (TmAtt1a, E); TmAttacin-1b (TmAtt1b, F); TmAttacin-2 (TmAtt2, G);TmDefensin-1 (TmDef1, H); TmDefensin-2 (TmDef2, I); TmColeptericin-1 (TmCole1, J); TmColeptericin-2 (TmCole2, K); TmCecropin-2 (TmCec2, L); TmTLP-1 (TmTLP1, M); and TmTLP2 (TmTLP-2, N). dsEGFP was used as a negative control and TmL27a was used as an internal control. The numbers above the bars indicate AMP mRNA expression levels. All experiments were repeated three times with similar results.
Figure 10.
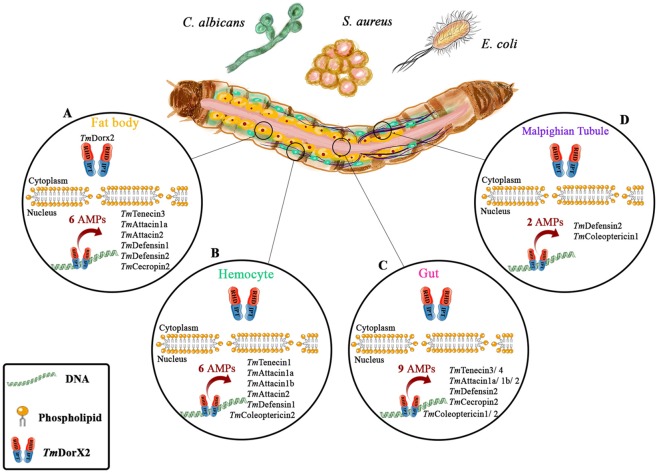
A schematic representation of the positive role of TmDorX2(downstream of Toll pathway) in controlling antimicrobial peptide (AMP) expression in the fat body (A), hemocytes (B)¸ gut (C), and Malpighian Tubules (D) of young larvae infected with E. coli, S. aureus, and C. albicans. In the fat body, 6 AMP-encoding genes are regulated by TmDorX2 upon fungal and bacterial infections (A). TmDorX2 affects the expression of TmTenecin-1, TmAttacin-1a, -1b, and -2, TmDefensin-1, and TmColeoptericin-2 in hemocytes in response to E. coli, S. aureus, and C. albicans infection. In the gut, 9 AMPs are downregulated in TmDorX2-silenced larvae, suggesting that TmDorX2 positively controls the induction of these genes (C). In the Malpighian Tubules, only 2 AMP-encoding genes, namely TmDefensin-2 and TmColeoptericin-1 are regulated by the Dorsal in T. molitor after microbial infection (D).
In general, after infection with all chosen microbes (E. coli, S. aureus, and C. albicans), the expression of several AMP genes was significantly decreased in TmDorX2-silenced larvae in comparison with dsEGFP-injected cohorts. Our present data demonstrate that TmAtt1a (Figs 7E and 8E) and TmAtt 2 (Figs 7G and 8G) were downregulated in hemocytes and the gut of TmDorX2-silenced larvae. Moreover, TmTene3 (Figs 6C and 8C), TmDef2 (Figs 6I, 8I and 9I) and TmCec2 (Figs 6L and 8L) were highly downregulated in the fat body, gut and MTs of dsTmDorX2 groups. Finally, in the hemocytes and gut, expression of TmCole2 (Figs 7K and 8K) and TmAtt1b (Figs 7F and 8F) in response to E. coli, S. aureus, and C. albicans was considerably decreased after TmDorX2 knockdown.
Discussion
Insects have evolved a robust tolerance and resistance mechanism against pathogenic infections which enable them to adapt to a wide variety of environmental niches (e.g., endoparasitic lifestyle)44. The plasticity of the innate immune defense mechanisms are pivotal towards combating microbial infection45. Towards elucidating the biochemical basis of innate immunity, such as the mechanism of pathogen recognition and the ensuing signaling cascades, D. melanogaster and T. castaneum have been used as reliable insect models. T. molitor has recently emerged as an excellent host-pathogen interaction model24. Compared to D. melanogaster, which is intolerant of high temperatures (25 °C and 37 °C)46, T. molitor exhibits thermal tolerance, making the species suitable for studying host defense mechanism against biotic and abiotic stressors47,48. In addition, laboratory rearing of T. molitor is relatively easy49 and its transcriptome, which represents the largest genetic sequence dataset for insects, has been already reported50, providing possibilities for carrying out molecular studies. A comparison of the Toll signaling pathway between T. molitor and Drosophila has revealed commonalities and differences in terms of the immune signaling mechanisms. In Drosophila, Lys-type PGNs of Gram-positive bacteria, β-1,3-glucan of fungi, and DAP-type PGNs of Gram-negative bacteria, activate the Toll signaling pathway51,52. Unlike Drosophila, the polymeric DAP-type PGN can also be recognized by the T. molitor PGRP-SA/GNBP1, complex leading to the sequential activation of a three-step proteolytic cascade, similar to that activated by Lys-Type PGN (the Imd pathway)53,54. Accordingly, in T. molitor, the recognition of bacterial and fungal PGN initiates Toll and Imd signaling pathways, which induce the expression of AMP genes23. Many intracellular proteins are present in the Toll signaling pathway. In the present study, we have focused on its final component, Dorsal, a transcription factor downstream the Toll pathway that translocates into the nucleus and binds to appropriate motifs in the promoters of specific AMP genes55.
Focusing on the T. molitor Toll pathway, we identified a Dorsal homolog using the T. castaneum Dorsal 2 as a query against the T. molitor RNAseq database. Conserved domain analysis of the full-length TmDorX2 ORF revealed RHD and IPT domains, and an NLS at the C-terminus of the IPT domain. All members of the NF-κB family share the structurally conserved RHD56. N-terminal sequences of RHD comprise a recognition loop that is responsible for DNA binding; the C-terminal sequences of RHD are required mainly for dimerization and interaction with inhibitor kappa Kinase (IKK)57. Previous studies on NF-κB dimerization found that the IPT domain is crucial for homodimerization, and deleting the IPT domains leads to the degradation of NF-κB precursors58,59. TmDorX2 is destined to translocate into the nucleus, hence, it contains an arginine (R)/lysine (K)-rich NLS (N-P307GALKRKREKY317-C)60. Sequence alignment of TmDorX2 RHD with that from other insects showed six conserved cysteine residues. The amino acid cysteine is fundamental for forming disulfide bonds, which is responsible for protein folding and stability.
The TmDorX2 mRNA levels were increased at the late larval and 2-day old pupa stages and reached peak values during adult stages, with the highest expression observed in 3-day-old adults. Prior studies on hormonal regulation of the innate immune response showed that juvenile hormone (JH) and ecdysone, which control development and growth in insects, modulate the expression of immune-induced genes in response to pathogen infection61. It is possible that, the fluctuations in the mRNA levels of TmDorX2 mRNA during different developmental stages are related to these versatile hormones.
TmDorX2 was expressed primarily in MTs and the fat body and less in hemocytes and the gut. Earlier studies in Drosophila have established the fat body (equivalent to the mammalian liver) as the foremost immune responsive organ that synthesizes and secretes AMPs into the hemolymph62,63. In addition to the fat body epithelial tissues, including the gut epithelium64, reproductive tract, trachea epithelial cells, and MTs (nephridia or kidney analogs)65,66, play an important role in immune defense. In the coleopteran model, Zophobas morio, the fat body and MTs are versatile tissues that share pivotal functions, such as immunity, detoxification, nitrogen metabolism, and eye pigmentation67. MTs are considered independent epithelial immune-responsive sites in insects. Furthermore, earlier studies have shown that genes involved in the Imd pathway are expressed in Drosophila MTs, and they lead to the induction of AMPs in response to microbial insults65,66. Moreover, Toll-associated transcripts, such as Toll receptors, Spz, Tube, Pelle, and Cactus have been detected in the MTs of Z. morio larvae68. MTs have also been regarded as immune sites that respond to ecdysone in the presence or absence of pathogenic microbes69. Furthermore, the mRNA levels of TmCactin, (positive regulator of Cactus degradation and mediator of Dorsal trans-nuclear localization) was found to be higher in MTs of T. molitor35. The results are indicative of simultaneous expression of TmCactin andTmDorX2 in MTs in response to bacterial challenges
To understand the involvement of Dorsal in T. molitor innate immunity, we examined the mRNA profiles of TmDorX2 upon E. coli, S. aureus, and C. albicans challenges. Our observation of increased TmDorX2 transcript levels in the whole-body, fat body, and hemocytes of the host larvae after infection with E. coli, and S. aureus is consistent with the findings in the Chinese shrimp, F. chinensis showing highly upregulated FcDorsal mRNA levels in response to both Gram-positive and Gram-negative bacteria16. In our study, the highest expression levels of TmDorX2 were observed 9 h post-infection in the immune tissues (fat body, hemocytes, and gut). Moreover, the increased TmDorX2 transcript levels in the hemocytes and gut of the E. coli-infected group in comparison with those in S. aureus and C. albicans-challenged groups, suggest that exposure to E. coli (Gram-negative bacteria) accelerates TmDorX2-Toll induction. A previous study on the expression of EsDorsal, in response to lipopolysaccharides (LPS) from E. coli, peptidoglycan (PG) from S. aureus, and zymosan (GLU) from Saccharomyces cerevisae, showed similar results wherein the EsDorsal responses to LPS were higher than those to GLU and PG19. As explained before in the case of Drosophila, the Toll signaling pathway senses β-1,3-glucan from fungi and lysine-type PGN from Gram-positive bacteria, whereas the Imd pathway is activated in response to DAP-type PGN from Gram-negative bacteria51. However, previous studies in T. molitor have shown that polymeric DAP-type PGN can be recognized by PGPRP-SA and GNBP1 of the Toll pathway23. Moreover, It has been recently reported that activation of the Toll pathway in T. molitor during E. coli infection can occur through TmToll-731. Based on these results, it is not surprising that TmDorX2 is highly expressed after E. coli challenge in T. molitor larvae.
To investigate the functional role of TmDorX2 in mediating humoral immunity through the expression of AMP-encoding genes in T. molitor, we first monitored the survival rates of young larvae treated with dsTmDorX2 upon E. coli, S. aureus, or C. albicans challenge. An RNAi efficiency of 91% was confirmed at day 2 post dsTmDorX2 injection. Larvae mortality rate was significantly higher after dsTmDorX2 silencing, reaching 48%, 42%, and 55% after 10 days of exposure to E. coli, S. aureus and C. albicans, respectively. This suggests that TmDorX2 has a positive and conserved role in T. molitor innate immunity against S. aureus and C. albicans infection, which is consistent with its role in the Pacific white shrimp, L. vannamei17. The highest mortality rate was observed in C. albicans-infected larvae compared to the E. coli- and S. aureus-challenged groups. It is possible that such high mortality rates of larvae after C. albicans insult are due to the absence of TmDorX2, which leads to lower AMP-encoding gene expression
AMPs are evolutionarily conserved effectors with bactericidal and antifungal activities, and are produced when free Dorsal translocates into the nucleus. qRT-PCR data from dsRNA-injected groups followed by pathogen infection were interpreted by describing TmDorX2 as a positive regulator when the expression of AMP genes was suppressed in dsTmDorX2-treated groups compared to dsEGFP-treated groups, and as a negative regulator in the opposite scenario. The expression of 11 AMP genes was highly increased in the fat body and gut of dsEGFP-injected larvae challenged with E. coli, whereas levels of all 11 AMP transcripts were significantly decreased in the dsTmDorX2-treated groups after E. coli insult. Among all 14 AMPs, the TmAttacin family70, TmTene271, and TmTene423 are well-known anti-Gram-negative AMPs. In the T. molitor gut, the expression levels of TmAtt1a, −1b and −2, and TmTene2 and −4 were dramatically downregulated in the dsTmDorX2-treated group. In the fat body and hemocytes of TmDorX2-silenced larvae, the levels of all mentioned anti-Gram-negative AMPs except for that of TmTene2 were downregulated. The expression levels of AMP genes in the fat body and gut of TmDorX2 knockdown larvae after E. coli insult (11 AMPs) were more strongly downregulated than those the expression of AMP genes in hemocytes and MTs (8 AMPs).
TmDorX2 knockdown decreased the survivability of larvae after C. albicans challenge. The increased susceptibility of the larvae can be explained by the fact that 11 AMP genes were markedly decreased in the gut of TmDorX2 dsRNA-treated T. molitor. More specifically, the expression of antifungal AMPs (TmTene3, TmTLP1, and TmTLP2) was strongly upregulated in response to C. albicans, and this response was greatly decreased upon TmDorX2 knockdown. The requirement of TmTene3 as an antifungal AMP is known72, and supports the results of our study. Furthermore, the mRNA levels of TmTene1 and TmTene2 observed upon TmDorX2 knockdown this study agree with previously reported results in the gut33. In the fat body and hemocytes, upon TmDorX2 knockdown, the expression of 10 and 8 AMP genes decreased after S. aureus injection, respectively. Taken together, our results suggest that the gut is a crucial immune tissue for mediating an innate immune response to C. albicans and E. coli, while the fat body is pivotal for conferring defense against S. aureus and E. coli.
Overall, the expression levels of AMP-encoding genes in TmDorX2 knockdown larvae significantly decreased after bacterial and fungal challenge compared with those in dsEGFP-injected groups. In a previous study, TmCactin knockdown led to the downregulation of 7AMP genes namely TmTene1 and −4, TmDef1 and −2, TmCole1 and −2, and TmAtt1b post-E. coli, -S. aureus, and -C. albicans challenges35. Upon infection with all the above mentioned microbes, dsTmDorX2-treated larvae showed significant downregulation of 6, 6, 9, and 2 AMP genes in the fat body, hemocytes, gut and MTs, respectively (Fig. 10). We must add that, similar to the depletion of TmCactin and TmToll-7 genes, TmDef2 is significantly decreased in the fat body, gut, and MTs tissues of dsTmDorX2-treated larvae31,35. In addition, we found that the induction of TmAtt2 was suppressed in the fat body, hemocytes, and gut of TmDorX2-silenced larvae; a similar downregulation has been observed in dsTmToll-7 injected larvae31. These findings suggest that TmDef2 and TmAtt2 are induced after Toll pathway stimulation mediated by E. coli, S. aureus, and C. albicans exposure.
Interestingly, we found several AMP genes induced in the dsTmDorX2-treated group compared to the dsEGFP-treated group. These results raise the possibility that TmDorX2 acts as a negative regulator of those AMP genes in different tissues, but also that of cross talk between Toll and another immune signaling pathway, such as the IMD pathway35.
Furthermore, ongoing investigations on another transcription factor, T. molitor Relish (TmRelish), showed that 9 AMP-encoding genes, namely TmTene2 and −4, TmDef1 and −2, TmCole1 and −2, TmAtt1a and −1b, and TmCec2, decreased in the fat body tissue of TmRelish-silenced larvae against same pathogens (unpublished data). Consequently, the mRNA levels of TmAtt1a, TmDef1 and −2, and TmCec2 are regulated by both TmRelish (Imd) and TmDorX2 (Toll) pathway in the fat body.
Finally, the mortality rate of T. molitor larvae upon C. albicans and S. aureus infection was higher that upon exposure to E. coli suggesting that TmDorX2 is required for mounting an innate immune response against S. aureus and C. albicans in the larval gut followed by a response in the fat body and hemocytes.
Conclusions
The Dorsal homologue identified in T. molitor (TmDorX2) was highly expressed in the fat body and MTs, and less in the hemocytes and gut. Upon challenge with E. coli, S. aureus, and C. albicans, the TmDorX2 mRNA levels were highly upregulated in the gut, fat body, and hemocytes of T. molitor larvae. According to mortality assay results, survival of TmDorX2-silenced larvae was remarkably decreased after S. aureus and C. albicans infection to a greater extent than after E. coli challenge, although the effect was significant in all infected groups. A Loss-of-function study of AMP expression revealed that TmDorX2 knockdown affects the induction of 11 AMP genes against E. coli and C. albicans in the larval gut, whereas it downregulates 10 AMP genes in response to S. aureus in the fat body. In summary, TmDorX2 can be considered as a positive regulator against E. coli, S. aureus, and C. albicans in the fat body, hemocytes, gut, and MTs of young T. molitor larvae. As inferred, injection of the microorganisms upregulated 6, 6, 9, and 2 AMP genes in fat body, hemocytes, gut and MTs of dsTmDorX2 larvae, respectively. TmDorX2 knockdown followed by microbial challenge resulted in high mortality of T. molitor larvae due to downregulation of AMPs, suggesting that TmDorX2 plays a key role against bacterial and fungal infections in immune tissues such as the fat body and gut.
Supplementary information
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (Grant No. 2018R1A2A2A05023367).
Author contributions
Han, Y.S. and Jo, Y.H. conceived and designed the experiments; Keshavarz, M., Park, K.B., Ko, H.J., and Edosa, T.T. performed the experiments; Jo, Y.H. and Keshavarz, M. analyzed the data; Han, Y.S., and Lee, Y.S. contributed reagents/ materials/analysis tools; Han, Y.S., Keshavarz, M. and Jo, Y.H. wrote the manuscript; Lee, Y.S. revised the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Maryam Keshavarz and Yong Hun Jo.
Supplementary information
is available for this paper at 10.1038/s41598-019-53497-4.
References
- 1.Sheehan G, Garvey A, Croke M, Kavanagh K. Innate humoral immune defences in mammals and insects: The same, with differences? Virulence. 2018;9:1625–1639. doi: 10.1080/21505594.2018.1526531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavine M, Strand M. Insect hemocytes and their role in immunity. Insect biochemistry and molecular biology. 2002;32:1295–1309. doi: 10.1016/S0965-1748(02)00092-9. [DOI] [PubMed] [Google Scholar]
- 3.Myllymäki H, Valanne S, Rämet M. The Drosophila imd signaling pathway. The Journal of Immunology. 2014;192:3455–3462. doi: 10.4049/jimmunol.1303309. [DOI] [PubMed] [Google Scholar]
- 4.Werner T, et al. A family of peptidoglycan recognition proteins in the fruit fly Drosophila melanogaster. Proceedings of the National Academy of Sciences. 2000;97:13772–13777. doi: 10.1073/pnas.97.25.13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshida H, Kinoshita K, Ashida M. Purification of a peptidoglycan recognition protein from hemolymph of the silkworm, Bombyx mori. Journal of Biological Chemistry. 1996;271:13854–13860. doi: 10.1074/jbc.271.23.13854. [DOI] [PubMed] [Google Scholar]
- 6.Lindsay SA, Wasserman SA. Conventional and non-conventional Drosophila Toll signaling. Developmental & Comparative Immunology. 2014;42:16–24. doi: 10.1016/j.dci.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleino A, Silverman N. The Drosophila IMD pathway in the activation of the humoral immune response. Developmental & Comparative Immunology. 2014;42:25–35. doi: 10.1016/j.dci.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh S, May MJ, Kopp EB. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annual review of immunology. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 9.Sagisaka A, Tanaka H, Furukawa S, Yamakawa M. Characterization of a homologue of the Rel/NF-κB transcription factor from a beetle, Allomyrina dichotoma. Biochimica et Biophysica Acta (BBA)-Gene Structure and Expression. 2004;1678:85–93. doi: 10.1016/j.bbaexp.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Steward R. Dorsal, an embryonic polarity gene in Drosophila, is homologous to the vertebrate proto-oncogene, c-rel. Science. 1987;238:692–694. doi: 10.1126/science.3118464. [DOI] [PubMed] [Google Scholar]
- 11.Shin SW, et al. REL1, a homologue of Drosophila dorsal, regulates toll antifungal immune pathway in the female mosquito Aedes aegypti. Journal of Biological Chemistry. 2005;280:16499–16507. doi: 10.1074/jbc.M500711200. [DOI] [PubMed] [Google Scholar]
- 12.Barillas‐Mury C, et al. Immune factor Gambif1, a new rel family member from the human malaria vector, Anopheles gambiae. The EMBO journal. 1996;15:4691–4701. doi: 10.1002/j.1460-2075.1996.tb00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lourenço, A. P., Florecki, M. M., Simões, Z. L. P. & Evans, J. D. Silencing of Apis mellifera dorsal genes reveals their role in expression of the antimicrobial peptide defensin‐1. Insect molecular biology (2018). [DOI] [PubMed]
- 14.Tanaka H, et al. A novel Rel protein and shortened isoform that differentially regulate antibacterial peptide genes in the silkworm Bombyx mori. Biochimica et Biophysica Acta (BBA)-Gene Structure and Expression. 2005;1730:10–21. doi: 10.1016/j.bbaexp.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 16.Li F, et al. A Dorsal homolog (FcDorsal) in the Chinese shrimp Fenneropenaeus chinensis is responsive to both bacteria and WSSV challenge. Developmental & Comparative Immunology. 2010;34:874–883. doi: 10.1016/j.dci.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Huang X-D, et al. Identification and functional study of a shrimp Dorsal homologue. Developmental & Comparative Immunology. 2010;34:107–113. doi: 10.1016/j.dci.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Cuthbertson BJ, BüLLESBACH EE, Fievet J, BACHèRE E, Gross PS. A new class (penaeidin class 4) of antimicrobial peptides from the Atlantic white shrimp (Litopenaeus setiferus) exhibits target specificity and an independent proline-rich-domain function. Biochemical Journal. 2004;381:79–86. doi: 10.1042/BJ20040330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu A-Q, et al. Molecular cloning and expression analysis of a dorsal homologue from Eriocheir sinensis. Developmental & Comparative Immunology. 2013;41:723–727. doi: 10.1016/j.dci.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Jeong C-B, et al. Identification and molecular characterization of dorsal and dorsal-like genes in the cyclopoid copepod Paracyclopina nana. Marine genomics. 2015;24:319–327. doi: 10.1016/j.margen.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Dorling J, Moraes C, Rolff J. Recognition, survival and persistence of Staphylococcus aureus in the model host Tenebrio molitor. Developmental & Comparative Immunology. 2015;48:284–290. doi: 10.1016/j.dci.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Dobson AJ, Johnston PR, Vilcinskas A, Rolff J. Identification of immunological expressed sequence tags in the mealworm beetle Tenebrio molitor. Journal of insect physiology. 2012;58:1556–1561. doi: 10.1016/j.jinsphys.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Chae J-H, et al. Purification and characterization of tenecin 4, a new anti-Gram-negative bacterial peptide, from the beetle Tenebrio molitor. Developmental & Comparative Immunology. 2012;36:540–546. doi: 10.1016/j.dci.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 24.de Souza PC, et al. Tenebrio molitor (Coleoptera: Tenebrionidae) as an alternative host to study fungal infections. Journal of microbiological methods. 2015;118:182–186. doi: 10.1016/j.mimet.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Roh K-B, et al. Proteolytic cascade for the activation of the insect toll pathway induced by the fungal cell wall component. Journal of Biological Chemistry, jbc. 2009;M109:007419. doi: 10.1074/jbc.M109.007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim C-H, et al. A three-step proteolytic cascade mediates the activation of the peptidoglycan-induced toll pathway in an insect. Journal of Biological Chemistry. 2008;283:7599–7607. doi: 10.1074/jbc.M710216200. [DOI] [PubMed] [Google Scholar]
- 27.Rolff, J. & Reynolds, S. Insect infection and immunity: evolution, ecology, and mechanisms. (Oxford university press, 2009).
- 28.An C, Jiang H, Kanost MR. Proteolytic activation and function of the cytokine Spätzle in the innate immune response of a lepidopteran insect, Manduca sexta. The FEBS journal. 2010;277:148–162. doi: 10.1111/j.1742-4658.2009.07465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber AN, et al. Binding of the Drosophila cytokine Spätzle to Toll is direct and establishes signaling. Nature immunology. 2003;4:794. doi: 10.1038/ni955. [DOI] [PubMed] [Google Scholar]
- 30.Kan H, et al. Molecular control of phenoloxidase-induced melanin synthesis in an insect. Journal of Biological Chemistry. 2008;283:25316–25323. doi: 10.1074/jbc.M804364200. [DOI] [PubMed] [Google Scholar]
- 31.Park S, et al. TmToll-7 plays a crucial role in innate immune responses against Gram-negative bacteria by regulating 5 AMP genes in Tenebrio molitor. Frontiers in Immunology. 2019;10:310. doi: 10.3389/fimmu.2019.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park J-W, et al. Clustering of peptidoglycan recognition protein-SA is required for sensing lysine-type peptidoglycan in insects. Proceedings of the National Academy of Sciences. 2007;104:6602–6607. doi: 10.1073/pnas.0610924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang YT, et al. Tenebrio molitor Gram‐negative‐binding protein 3 (TmGNBP3) is essential for inducing downstream antifungal Tenecin 1 gene expression against infection with Beauveria bassiana JEF‐007. Insect science. 2018;25:969–977. doi: 10.1111/1744-7917.12482. [DOI] [PubMed] [Google Scholar]
- 34.Patnaik BB, et al. Gene structure, cDNA characterization and RNAi-based functional analysis of a myeloid differentiation factor 88 homolog in Tenebrio molitor larvae exposed to Staphylococcus aureus infection. Developmental & Comparative Immunology. 2014;46:208–221. doi: 10.1016/j.dci.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 35.Jo YH, et al. TmCactin plays an important role in Gram-negative and-positive bacterial infection by regulating expression of 7 AMP genes in Tenebrio molitor. Scientific reports. 2017;7:46459. doi: 10.1038/srep46459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones P, et al. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30:1236–1240. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mount, D. W. Using the basic local alignment search tool (BLAST). Cold Spring Harbor Protocols, 2007, pdb. top17 (2007). [DOI] [PubMed]
- 38.Kosugi S, Hasebe M, Tomita M, Yanagawa H. Systematic identification of cell cycle-dependent yeast nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proceedings of the National Academy of Sciences. 2009;106:10171–10176. doi: 10.1073/pnas.0900604106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larkin MA, et al. Clustal W and Clustal X version 2.0. bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 40.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular biology and evolution. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Bioinformatics. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- 42.Yaffe H, et al. LogSpin: a simple, economical and fast method for RNA isolation from infected or healthy plants and other eukaryotic tissues. BMC research notes. 2012;5:45. doi: 10.1186/1756-0500-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C T method. Nature protocols. 2008;3:1101. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 44.Miller CV, Cotter SC. Resistance and tolerance: the role of nutrients on pathogen dynamics and infection outcomes in an insect host. Journal of Animal Ecology. 2018;87:500–510. doi: 10.1111/1365-2656.12763. [DOI] [PubMed] [Google Scholar]
- 45.Urbański A, Adamski Z, Rosiński G. Developmental changes in haemocyte morphology in response to Staphylococcus aureus and latex beads in the beetle Tenebrio molitor L. Micron. 2018;104:8–20. doi: 10.1016/j.micron.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Linder JE, Owers KA, Promislow DE. The effects of temperature on host–pathogen interactions in D. melanogaster: Who benefits? Journal of insect physiology. 2008;54:297–308. doi: 10.1016/j.jinsphys.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevens MM, Jackson S, Bester SA, Terblanche JS, Chown SL. Oxygen limitation and thermal tolerance in two terrestrial arthropod species. Journal of Experimental Biology. 2010;213:2209–2218. doi: 10.1242/jeb.040170. [DOI] [PubMed] [Google Scholar]
- 48.Li D-D, et al. Using Galleria mellonella–Candida albicans infection model to evaluate antifungal agents. Biological and Pharmaceutical Bulletin. 2013;36:1482–1487. doi: 10.1248/bpb.b13-00270. [DOI] [PubMed] [Google Scholar]
- 49.Osimani A, et al. The bacterial biota of laboratory-reared edible mealworms (Tenebrio molitor L.): from feed to frass. International journal of food microbiology. 2018;272:49–60. doi: 10.1016/j.ijfoodmicro.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 50.Oppert B, et al. Transcriptome profiling of the intoxication response of Tenebrio molitor larvae to Bacillus thuringiensis Cry3Aa protoxin. PloS one. 2012;7:e34624. doi: 10.1371/journal.pone.0034624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanji T, Ip YT. Regulators of the Toll and Imd pathways in the Drosophila innate immune response. Trends in immunology. 2005;26:193–198. doi: 10.1016/j.it.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 52.Liu Y, Zhao X, Huang J, Chen M, An J. Structural Insights into the Preferential Binding of PGRP-SAs from Bumblebees and Honeybees to Dap-Type Peptidoglycans Rather than Lys-Type Peptidoglycans. The Journal of Immunology. 2019;202:249–259. doi: 10.4049/jimmunol.1800439. [DOI] [PubMed] [Google Scholar]
- 53.Yu Y, et al. Diversity of innate immune recognition mechanism for bacterial polymeric meso-diaminopimelic acid-type peptidoglycan in insects. Journal of Biological Chemistry. 2010;285:32937–32945. doi: 10.1074/jbc.M110.144014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryu K, Park J, Kurokawa K, Matsushita M, Lee B. The molecular activation and regulation mechanisms of proteolytic Toll signaling cascade in insect innate immunity. Invertebrate Survival. Journal. 2010;7:181–191. [Google Scholar]
- 55.Hwang J, Park Y, Kim Y, Hwang J, Lee D. An entomopathogenic bacterium, Xenorhabdus nematophila, suppresses expression of antimicrobial peptides controlled by Toll and Imd pathways by blocking eicosanoid biosynthesis. Archives of insect biochemistry and physiology. 2013;83:151–169. doi: 10.1002/arch.21103. [DOI] [PubMed] [Google Scholar]
- 56.Oeckinghaus A, Ghosh S. The NF-κB family of transcription factors and its regulation. Cold Spring Harbor perspectives in biology. 2009;1:a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghosh S, Dass JFP. Non-canonical pathway network modelling and ubiquitination site prediction through homology modelling of NF-κB. Gene. 2016;581:48–56. doi: 10.1016/j.gene.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 58.Lin L, DeMartino GN, Greene WC. Cotranslational biogenesis of NF-κB p50 by the 26S proteasome. Cell. 1998;92:819–828. doi: 10.1016/S0092-8674(00)81409-9. [DOI] [PubMed] [Google Scholar]
- 59.Rape M, et al. Mobilization of processed, membrane-tethered SPT23 transcription factor by CDC48UFD1/NPL4, a ubiquitin-selective chaperone. Cell. 2001;107:667–677. doi: 10.1016/S0092-8674(01)00595-5. [DOI] [PubMed] [Google Scholar]
- 60.Lange A, et al. Classical nuclear localization signals: definition, function, and interaction with importin α. Journal of Biological Chemistry. 2007;282:5101–5105. doi: 10.1074/jbc.R600026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Flatt T, et al. Hormonal regulation of the humoral innate immune response in Drosophila melanogaster. Journal of Experimental Biology. 2008;211:2712–2724. doi: 10.1242/jeb.014878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manfruelli P, Reichhart JM, Steward R, Hoffmann JA, Lemaitre B. A mosaic analysis in <em>Drosophila</em> fat body cells of the control of antimicrobial peptide genes by the Rel proteins Dorsal and DIF. The EMBO Journal. 1999;18:3380–3391. doi: 10.1093/emboj/18.12.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bettencourt R, Asha H, Dearolf C, Ip YT. Hemolymph‐dependent and‐independent responses in Drosophila immune tissue. Journal of cellular biochemistry. 2004;92:849–863. doi: 10.1002/jcb.20123. [DOI] [PubMed] [Google Scholar]
- 64.Huang JH, Jing X, Douglas AE. The multi-tasking gut epithelium of insects. Insect Biochem Mol Biol. 2015;67:15–20. doi: 10.1016/j.ibmb.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng W, et al. Dehydration triggers ecdysone-mediated recognition-protein priming and elevated anti-bacterial immune responses in Drosophila Malpighian tubule renal cells. BMC biology. 2018;16:60. doi: 10.1186/s12915-018-0532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McGettigan J, et al. Insect renal tubules constitute a cell-autonomous immune system that protects the organism against bacterial infection. Insect biochemistry and molecular biology. 2005;35:741–754. doi: 10.1016/j.ibmb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 67.Silva JR, Amaral DT, Viviani VR. Comparison of the Malpighian tubules and fat body transcriptional profiles of Zophobas morio larvae (Coleoptera: Tenebrionidae) Comparative Biochemistry and Physiology Part D: Genomics and Proteomics. 2019;29:95–105. doi: 10.1016/j.cbd.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 68.Silva, J. R., Amaral, D. T. & Viviani, V. R. Comparison of the Malpighian tubules and fat body transcriptional profiles of Zophobas morio larvae (Coleoptera: Tenebrionidae). Comparative Biochemistry and Physiology Part D: Genomics and Proteomics, (2018). [DOI] [PubMed]
- 69.Verma P, Tapadia MG. Early gene Broad complex plays a key role in regulating the immune response triggered by ecdysone in the Malpighian tubules of Drosophila melanogaster. Molecular immunology. 2015;66:325–339. doi: 10.1016/j.molimm.2015.03.249. [DOI] [PubMed] [Google Scholar]
- 70.Jo YH, et al. In silico identification, characterization and expression analysis of attacin gene family in response to bacterial and fungal pathogens in Tenebrio molitor. Entomological research. 2018;48:45–54. doi: 10.1111/1748-5967.12287. [DOI] [Google Scholar]
- 71.Moon HJ, Lee SY, Kurata S, Natori S, Lee BL. Purification and Molecular Cloning of cDNA for an Inducible Antibacterial Protein from Larvae of the Coleopteran, Tenebrio molitor1. The Journal of Biochemistry. 1994;116:53–58. doi: 10.1093/oxfordjournals.jbchem.a124502. [DOI] [PubMed] [Google Scholar]
- 72.Maistrou S, et al. A constitutively expressed antifungal peptide protects Tenebrio molitor during a natural infection by the entomopathogenic fungus Beauveria bassiana. Developmental & Comparative Immunology. 2018;86:26–33. doi: 10.1016/j.dci.2018.04.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.