Abstract
Background
To evaluate the association between retinal artery occlusion (RAO) and subclinical coronary artery disease (CAD).
Methods
We studied 41 patients with non-arteritic RAO without any history or symptoms of CAD, who had undergone coronary computed tomographic angiography (CCTA) for systemic atherosclerotic evaluation between 2007 and 2012. The age- and gender-matched control group comprised 4-fold subjects who were randomly selected from asymptomatic subjects who underwent CCTA during general health evaluation. Medical records and CCTA findings were compared between RAO patients and control groups. Multiple logistic regression analysis was carried out to assess the risk factors associated with CAD.
Results
Cardiovascular risk factors were not significantly different between RAO patients and control groups. RAO patients showed higher coronary artery calcium score than did control subjects (267.9 ± 674.9 vs. 120.2 ± 289.5). On CCTA, the prevalence of obstructive CAD (diameter stenosis ≥ 50%) in RAO patients was significantly higher than that in controls (29% vs. 15%; odds ratio [OR], 3.0). RAO patients demonstrated a significantly higher segment-involvement score (SIS) (2.6 ± 3.0 vs. 1.6 ± 2.4) and segment-stenosis score (SSS) (3.6 ± 4.8 vs. 2.0 ± 3.3) than did controls. After adjustment of associated factors, RAO showed significant association (OR, 3.0) with obstructive CAD and extensive CAD (SIS > 4: OR, 2.8; SSS > 8: OR, 3.4).
Conclusion
Patients with RAO had a higher prevalence of subclinical obstructive CAD with a more extensive and heavier burden of coronary artery plaques than did age- and gender-matched controls. Physicians should understand the potential risk of CAD in RAO patients.
Keywords: Atherosclerosis, Coronary Artery Disease, Coronary Computed Tomographic Angiography, Retinal Artery Occlusion
Graphical Abstract
INTRODUCTION
Retinal artery occlusion (RAO) is one of the leading causes of sudden and catastrophic visual loss.1 Because the pathogenesis and incidence pattern of RAO are similar to those of ischemic stroke,2 RAO is often regarded as another sign of cerebrovascular disease that may require systemic evaluation and appropriate general care.1,3 It has been reported that RAO is associated with increased risk for subsequent stroke4,5,6 and the presence of carotid artery stenosis.7 Furthermore, given that cardiovascular risk factors are commonly observed in RAO patients,7 RAO is expected to be associated with the presence of coronary artery disease (CAD) as well as carotid artery stenosis and ischemic stroke. However, to date, limited data are available that suggests an increased occurrence of acute coronary syndrome after the diagnosis of RAO.8 The prevalence, severity, and extent of CAD at the time of diagnosis of RAO has never been studied before.
The need for systemic evaluations of cardiovascular risks in RAO patients is increasing, while explicit evidence-based guidance is lacking.9 Although there was general consensus about the importance of carotid artery screening, cardiac evaluation is only limited to electrocardiography or echocardiography without evaluation of CAD.9 Coronary computed tomographic angiography (CCTA) has been widely utilized for the noninvasive evaluation of CAD, providing excellent diagnostic and prognostic value.10 Compared to traditional approaches for assessing CAD based on the evaluation of myocardial ischemia, CCTA directly visualizes coronary atherosclerosis and provides important information, including presence, severity, and extent of CAD.11,12 Although CCTA screening of asymptomatic patients is currently under debate and was graded as uncertain,13 simultaneous evaluation of CAD and the source of embolism using CCTA is deemed potentially useful for patients with ischemic stroke.14 Considering the common pathophysiology between RAO and ischemic stroke, CCTA could also be useful for patients with RAO.
In the present case-control study, we aimed to assess using CCTA whether asymptomatic RAO patients are at increased risk of subclinical CAD compared with control subjects.
METHODS
Study population
Patients with newly detected non-arteritic RAO who underwent CCTA for the evaluation of systemic vascular status within 12 months at Seoul National University Bundang Hospital (SNUBH) between January 2007 and December 2012 were retrospectively assessed for eligibility. CCTA was recommended in most newly visiting patients who had not previously been evaluated for heart disease, and was performed only in the patients who had been informed and agreed to the exam. Patients were excluded from the study if any of the following was present: 1) iatrogenic or traumatic RAOs, such as those due to accidental intravascular facial filler injection or due to orbital trauma, 2) arteritic RAO, 3) combined retinal vein occlusion, and 4) known or suspected CAD at the time of RAO diagnosis. Of the 41 RAO patients enrolled, 32 had central RAO (CRAO) and 9 had branch RAO (BRAO). For the comparison of the prevalence and severity of subclinical CAD to the healthy population, we also retrospectively formed age- and gender-matched control group. The age- and gender-matched control group consisted of a 4-fold number of subjects (n = 164), who were randomly selected from the asymptomatic people without known or suspected CADs who underwent CCTA as a part of general health evaluation at the Health Promotion Center of SNUBH. Fundus retinal photography was performed in most of the healthy participants (n = 152/164; 93%) as a part of general health evaluation, and no RAO was found in any of the fundus photography or medical records. The detailed reading results are as follows: 127 subjects with normal fundus; 10 subjects with age-related macular degeneration; 9 subjects with glaucoma or glaucomatous optic disc; 3 subjects with idiopathic epiretinal membrane; 2 subjects with branch retinal vein occlusion, 1 subject with nonproliferative diabetic retinopathy.
CCTA data acquisition and image analysis
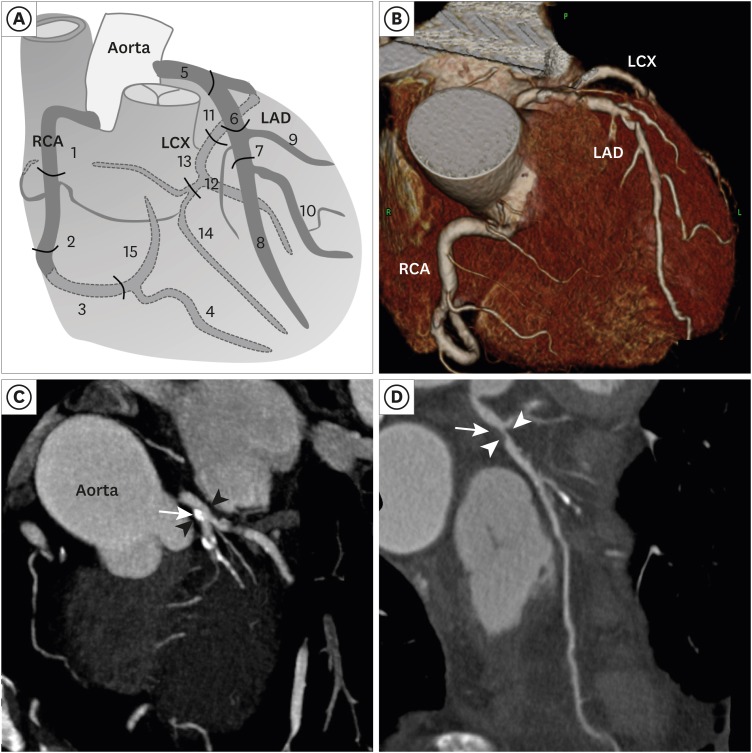
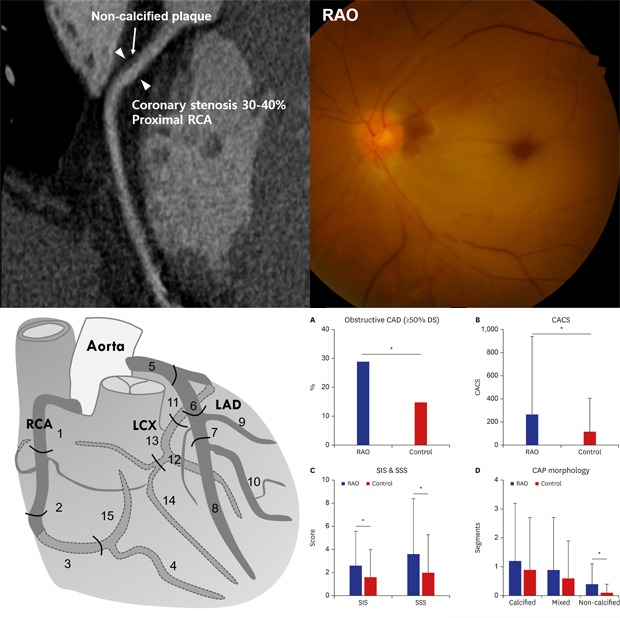
Unenhanced and contrast-enhanced CCTA were performed using a multi-detector CT scanner (Brilliance 64 or Brilliance iCT; Philips Medical Systems, Best, The Netherlands), in accordance with established guidelines and the institutional protocols at the time of the scan.15 CCTA images were transferred to an offline 3-dimensional (3D) workstation and independently analyzed by a cardiac radiologist. Coronary artery calcium score (CACS) was measured as previously described by Agatston et al.16 Based on the CACS, the study participants were categorized in the following manner; 0, 1–99, 100–399, and ≥ 400. The presence, severity, and composition of coronary atherosclerotic plaques were evaluated with a per-segment analysis, according to the modified American Heart Association 15 segment criteria.17 A schematic drawing of the segmentation and 3D reconstructed image of the coronary arteries are shown in Fig. 1. Coronary atherosclerotic plaque was defined as a structure clearly distinct from the lumen and surrounding tissue, and measuring > 1 mm2 within and/or adjacent to the vessel lumen. Patients were categorized as having no CAD, non-obstructive CAD (plaque < 50% diameter stenosis [DS]), and obstructive CAD (plaque ≥ 50% DS). Because coronary atherosclerotic burden as well as obstruction severity is an important prognostic factor associated with adverse cardiovascular events,18,19 coronary atherosclerotic plaque burden was evaluated using the segment-involvement score (SIS) and the segment-stenosis score (SSS).19 The SIS was defined as the total number of coronary artery segments with coronary atherosclerotic plaque, irrespective of the degree of DS, the total ranging from 0 to 15. The SSS was calculated as the sum of the score of each segment graded as 0–2 (0, no coronary artery plaque [CAP]; 1, CAP < 50% DS; 2, CAP ≥ 50% DS) ranging from 0 to 30. Plaque composition was classified as non-calcified (< 30% calcified plaque volume), mixed (30% to 70%), or calcified (> 70%), according to the calcified component (> 130 Hounsfield Units).15,20 Representative CCTA images of coronary artery stenosis and plaques in patients with RAO are shown in Fig. 1.
Fig. 1. The anatomy of the coronary arteries and the representative CCTA images of coronary artery disease. (A) A schematic drawing of the anatomy and segmentation of the coronary arteries. Fifteen segments are as follows: 1 - proximal RCA; 2 - middle RCA; 3 - distal RCA; 4 - posterior descending; 5 - left main; 6 - proximal LAD; 7 - middle LAD; 8 - distal LAD; 9 - diagonal 1; 10 - diagonal 2; 11 - proximal LCX; 12 - ob marginal; 13 - distal LCX; 14 - ob marginal 2; 15 - posterolateral branch. (B) Three-dimensional reconstruction of CCTA image in a RAO patient. (C) CCTA showed 20%–30% of stenosis (arrow heads) at left main coronary artery due to calcified plaque (arrow) in a 68-year-old man with central RAO. (D) CCTA showed severe stenosis (arrow heads) at proximal LAD due to non-calcified plaque (arrow) in a 72-year-old man with central RAO.
RAO = retinal artery occlusion, CCTA = coronary computed tomographic angiography, RCA = right coronary artery, LCX = left circumflex artery, LAD = left anterior descending.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation, whereas categorical variables are presented as absolute values and their percentages. Differences between continuous variables were analyzed by the unpaired student t-test, and those between categorical variable by the χ2 test or Fisher's exact test, as appropriate. We performed a subgroup analysis comparing BRAO and CRAO patients with each age- and gender-matched control. Multiple logistic regression analysis (backward elimination) was conducted to identify independent predictors of the CACS > 0, subclinical CAD of ≥ 50% DS, SIS > 4, and SSS > 8 on CCTA findings. Conventional risk factors including age, gender, hypertension, diabetes, dyslipidemia, current smoking, obesity, and RAO were considered in the multivariable analysis. All statistical analyses were performed using SPSS 22.0 (IBM, Chicago, IL, USA).
Ethics statement
This retrospective study was performed in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of the Seoul National University Bundang Hospital (IRB B-1811/504-102). The requirement of informed consent was waived from the IRB.
RESULTS
Demographic data and cardiovascular risk factors
Baseline characteristics of 41 RAO patients (30 men, 11 women; mean age, 61.7 ± 15.1 years) were compared to the age- and gender-matched 4-fold controls (120 men, 44 women; mean age, 62.1 ± 14.6 years). There was no significant difference in baseline demographics and clinical features between RAO patients and asymptomatic control subjects, such as age, gender, and conventional cardiovascular risk factors including hypertension, diabetes mellitus, dyslipidemia, obesity, and smoking history (Table 1). We also divided RAO patients into BRAO and CRAO subgroups and compared them with each corresponding age- and gender-matched control (Supplementary Table 1). There were no significant differences in conventional risk factors when the CRAO and BRAO groups were compared to each control group. However, the proportion of current smokers was higher in the CRAO group compared to the corresponding control group (28% vs. 11%; P = 0.045, linear-by-linear association).
Table 1. Baseline clinical characteristics and CCTA findings of RAO patients and controls.
| Variables | RAO (n = 41) | Control (n = 164) | P value | ||
|---|---|---|---|---|---|
| Central RAO | 32 (78) | ||||
| Age, yr | 61.7 ± 15.1 | 62.1 ± 14.6 | 0.881 | ||
| Gender | > 0.999 | ||||
| Men | 30 (73) | 120 (73) | > 0.999 | ||
| Women | 11 (27) | 44 (27) | |||
| Comorbidity | |||||
| Hypertension | 24 (59) | 73 (45) | 0.108 | ||
| Diabetes mellitus | 9 (22) | 31 (19) | 0.660 | ||
| Dyslipidemia | 22 (54) | 77 (47) | 0.442 | ||
| Obesitya | 12 (29) | 50 (31) | 0.879 | ||
| Smoking | 0.072b | ||||
| Current smoker | 11 (27) | 25 (15) | |||
| Ex-smoker | 7 (17) | 26 (16) | |||
| Never | 23 (56) | 113 (69) | |||
| CCTA | |||||
| CACS | 267.9 ± 674.9 | 120.2 ± 289.5 | 0.034 | ||
| CACS category | 0.159b | ||||
| 0 | 17 (41) | 78 (48) | |||
| 1–99 | 10 (24) | 48 (29) | |||
| 100–399 | 7 (17) | 23 (14) | |||
| ≥ 400 | 7 (17) | 15 (9) | |||
| CAD (obstructive or non-obstructive) | 25 (61) | 87 (53) | 0.362 | ||
| Obstructive CAD (≥ 50 DS) | 12 (29) | 24 (15) | 0.028 | ||
| SISc | 2.6 ± 3.0 | 1.6 ± 2.4 | 0.032 | ||
| SSSd | 3.6 ± 4.8 | 2.0 ± 3.3 | 0.016 | ||
| Subjects with SIS >4 | 10 (24) | 17 (10) | 0.018 | ||
| Subjects with SSS >8 | 7 (17) | 10 (6) | 0.023 | ||
| By CAP morphology | |||||
| Segments with any calcified plaque | 1.2 ± 2.0 | 0.9 ± 1.8 | 0.359 | ||
| Segments with any mixed plaque | 0.9 ± 1.8 | 0.6 ± 1.3 | 0.193 | ||
| Segments with any non-calcified plaque | 0.4 ± 0.7 | 0.1 ± 0.3 | < 0.001 | ||
Data are presented as mean ± standard deviation or number (%). P < 0.05 was deemed to indicate clinical significance, values in boldface are statistically significant.
CCTA = coronary computed tomographic angiography, RAO = retinal artery occlusion, CACS = coronary artery calcium score, CAD = coronary artery disease, DS = diameter stenosis, SIS = segment-involvement score, SSS = segment-stenosis score, CAP = coronary artery plaque.
aObesity indicates body mass index ≥ 25.0 kg/m2, bLinear-by-linear association; cSIS: total number of coronary artery segments with exhibiting plaque, ranging from 0 to 15; dSSS: sum of the score of each segment graded as 0–2, ranging from 0 to 30 (0, no CAP; 1, < 50 DS; 2, ≥ 50 DS).
CCTA: comparison between patient and control groups
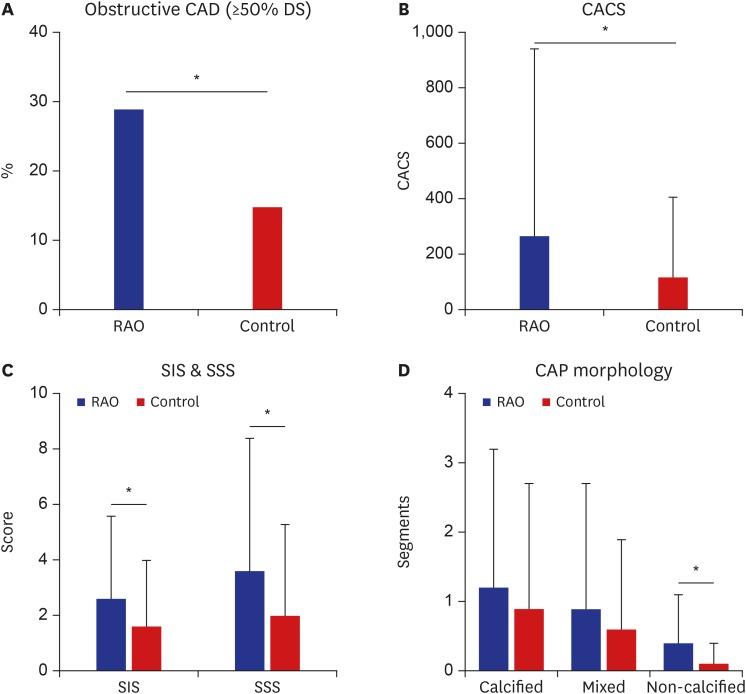
The detailed CCTA findings of RAO patients and control subjects are summarized in Table 1. Mean CACS of the 41 RAO patients was significantly higher than that of the control subjects (267.9 ± 674.9 vs. 120.2 ± 289.5; P = 0.034, unpaired t-test). Although the overall prevalence of any CAD including both non-obstructive and obstructive CAD as evidenced by CCTA was not significantly different in RAO patients compared to control subjects (61% vs. 53%; P = 0.362, χ2 test), obstructive CAD was more commonly observed in RAO patients compared to control subjects (29% vs. 15%; P = 0.028). Both SIS and SSS were significantly higher in RAO patients compared to control subjects (SIS, 2.6 ± 3.0 vs. 1.6 ± 2.4; P = 0.032, and SSS, 3.6 ± 4.8 vs. 2.0 ± 3.3; P = 0.016) suggesting the more extensive and heavier burden of subclinical CAD in RAO patients. The number of subjects with SIS > 4 was significantly higher in RAO patients (n = 10/41; 24%) than in control groups (n = 17/164, 10%; P for comparison = 0.018). The number of subjects with SSS > 8 was also significantly higher in RAO patients (n = 7/41; 17%) than in control groups (n = 10/164; 6%; P for comparison = 0.023). As for plaque composition, while the number of segments with calcified or mixed plaques were not significantly different, the number of non-calcified plaques was significantly higher in RAO patients (0.4 ± 0.7 vs. 0.1 ± 0.3; P < 0.001) (Fig. 2).
Fig. 2. Comparison of coronary computed tomographic angiography findings between RAO patients and control groups. (A) Obstructive CAD was significantly common in RAO patients compared to control subjects. (B) Mean coronary artery calcium score of RAO patients was significantly higher than that of control subjects. (C) Both SIS and SSS were significantly higher in RAO patients compared to control subjects. (D) The number of segments with calcified or mixed plaques were not significantly different; however, the number of non-calcified plaques was significantly higher in RAO patients.
RAO = retinal artery occlusion, CAD = coronary artery disease, DS = diameter stenosis, CACS = coronary artery calcium score, SIS = segment-involvement score, SSS = segment-stenosis score, CAP = coronary artery plaque.
*Statistically significant difference, P value < 0.05.
Clinical risk factors associated with CAD and CAPs: multiple logistic regression analysis
We evaluated clinical risk factors associated with CAD identified by CCTA (Table 2). RAO (odds ratio [OR], 3.0; 95% confidence interval [CI], 1.2–7.6, P = 0.024) as well as older age (≥ 70 years; OR, 6.2; 95% CI, 2.6–14.7; P < 0.001), men gender (OR, 4.3; 95% CI, 1.4–13.3; P = 0.012), and diabetes (OR, 6.2; 95% CI, 2.5–15.1; P < 0.001) were associated with the presence of obstructive CAD (≥ 50% DS). For the presence of CAD with SIS > 4 (criteria for extensive CAD), RAO (OR, 2.8; 95% CI, 1.1–7.0; P = 0.031) and older age (≥ 70 years; OR, 3.0; 95% CI, 1.2–7.1; P = 0.015) were significant predictors. CAD with SSS > 8 (criteria for significant CAP burden) was significantly associated with RAO (OR, 3.4; 95% CI, 1.1–10.3; P = 0.030), older age (≥ 70 years; OR, 3.8; 95% CI, 1.3–11.4; P = 0.015), and diabetes (OR, 4.0; 95% CI, 1.4–11.8; P = 0.011). Among RAO subgroups, CRAO showed greater association with obstructive CAD, SIS > 4, and SSS > 8 than did BRAO (CRAO vs. control; OR, 3.6, 3.0, and 3.8, respectively) (Table 3).
Table 2. Uni- and multi-variable analysis for obstructive CAD (≥ 50% DS), SIS > 4, and SSS >8 based on findings on coronary computed tomographic angiography.
| Variables | Obstructive CAD (≥ 50% DS) | SISa > 4 | SSSb > 8 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | Univariable | Multivariable | |||||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Age ≥ 70 yr | 6.2 (2.5–15.3) | < 0.001 | 6.2 (2.6–14.7) | 0.000 | 3.3 (1.3–8.2) | 0.010 | 3.0 (1.2–7.1) | 0.015 | 4.8 (1.5–15.4) | 0.008 | 3.8 (1.3–11.4) | 0.015 |
| Gender (men) | 5.8 (1.8–19.3) | 0.004 | 4.3 (1.4–13.3) | 0.012 | 1.8 (0.6–5.6) | 0.276 | 2.2 (0.9–5.6) | 0.089 | 3.2 (0.7–14.2) | 0.119 | - | - |
| Hypertension | 1.4 (0.6–3.3) | 0.499 | - | - | 2.5 (1.0–6.4) | 0.062 | - | - | 2.2 (0.7–7.0) | 0.204 | - | - |
| DM | 5.7 (2.3–14.3) | 0.000 | 6.2 (2.5–15.1) | < 0.001 | 2.3 (0.9–5.8) | 0.084 | 2.4 (1.0–6.1) | 0.060 | 3.4 (1.1–10.3) | 0.028 | 4.0 (1.4–11.8) | 0.011 |
| Dyslipidemia | 1.6 (0.7–3.8) | 0.289 | - | - | 1.4 (0.6–3.5) | 0.423 | - | - | 1.4 (0.4–4.1) | 0.595 | - | - |
| Current smoker | 0.4 (0.1–1.3) | 0.123 | - | - | 1.2 (0.4–4.1) | 0.716 | - | - | 0.6 (0.1–3.0) | 0.559 | - | - |
| Obesity | 0.9 (0.3–2.3) | 0.809 | - | - | 0.9 (0.3–2.3) | 0.747 | - | - | 1.6 (0.5–5.2) | 0.406 | - | - |
| RAO | 3.2 (1.2–8.6) | 0.019 | 3.0 (1.2–7.6) | 0.024 | 2.6 (1.0–6.8) | 0.052 | 2.8 (1.1–7.0) | 0.031 | 3.5 (1.1–11.3) | 0.036 | 3.4 (1.1–10.3) | 0.030 |
P < 0.05 was deemed to indicate clinical significance, values in boldface are statistically significant.
CAD = coronary artery disease, DS = diameter stenosis, SIS = segment-involvement score, SSS = segment-stenosis score, OR = odds ratio, CI = confidence interval, DM = diabetes mellitus, RAO = retinal artery occlusion.
aSIS, total number of coronary artery segments with exhibiting plaque, ranging from 0 to 15; bSSS, sum of the score of each segment graded as 0–2, ranging from 0 to 30 (0, no CAP; 1, < 50% DS; 2, ≥ 50% DS).
Table 3. Subgroup analysis according to central or branch RAO. Uni- and multi-variable analysis for obstructive CAD (≥ 50% DS), SIS > 4, and SSS > 8 based on findings on coronary computed tomographic angiography.
| Variables | Obstructive CAD (≥ 50% DS) | SISa > 4 | SSSb > 8 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | Univariable | Multivariable | ||||||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age ≥ 70 yr | 4.5 (2.1–9.5) | < 0.001 | 5.5 (2.3–13.1) | < 0.001 | 3.3 (1.4–7.5) | < 0.001 | 2.9 (1.2–7.0) | 0.017 | 3.9 (1.4–11.1) | 0.010 | 3.5 (1.2–10.4) | 0.027 | |
| Gender (men) | 2.6 (1.0–7.1) | 0.061 | 4.6 (1.4–14.3) | 0.010 | 1.3 (0.5–3.5) | 0.563 | - | - | 1.8 (0.5–6.5) | 0.378 | - | - | |
| Hypertension | 2.0 (0.9–4.1) | 0.071 | 1.4 (0.6–3.4) | 0.420 | 3.0 (1.3–7.3) | 0.013 | 2.2 (0.9–5.6) | 0.090 | 2.9 (1.0–8.6) | 0.053 | 2.0 (0.6–6.2) | 0.247 | |
| DM | 5.7 (2.6–12.5) | < 0.001 | 5.2 (2.1–12.9) | < 0.001 | 2.9 (1.2–7.0) | 0.017 | 2.4 (0.9–6.0) | 0.073 | 4.3 (1.6–12.1) | 0.005 | 3.4 (1.1–10.0) | 0.029 | |
| Dyslipidemia | 1.6 (0.8–3.4) | 0.187 | - | - | 1.7 (0.7–3.8) | 0.224 | - | - | 1.6 (0.6–4.4) | 0.368 | - | - | |
| Current smoker | 0.7 (0.3–2.0) | 0.525 | - | - | 1.4 (0.5–3.8) | 0.496 | - | - | 1.0 (0.3–3.7) | 0.992 | - | - | |
| Obesity | 0.7 (0.3–1.7) | 0.452 | - | - | 0.8 (0.3–2.0) | 0.601 | - | - | 1.3 (0.4–3.6) | 0.637 | - | - | |
| RAO | |||||||||||||
| Branch RAO | - | - | - | - | 1.1 (0.1–9.2) | 0.943 | 1.8 (0.2–17.3) | 0.593 | - | - | - | - | |
| Central RAO | 3.5 (1.5–8.1) | 0.003 | 3.6 (1.3–9.7) | 0.011 | 3.4 (1.3–8.5) | 0.009 | 3.0 (1.1–7.8) | 0.028 | 4.3 (1.5–12.4) | 0.007 | 3.8 (1.3–11.8) | 0.019 | |
P < 0.05 was deemed to indicate clinical significance, values in boldface are statistically significant.
CAD = coronary artery disease, DS = diameter stenosis, SIS = segment-involvement score, SSS = segment-stenosis score, OR = odds ratio, CI = confidence interval, DM = diabetes mellitus, RAO = retinal artery occlusion.
aSIS, total number of coronary artery segments with exhibiting plaque, ranging from 0 to 15; bSSS, sum of the score of each segment graded as 0–2, ranging from 0 to 30 (0, no CAP; 1, < 50% DS; 2, ≥ 50% DS).
DISCUSSION
We performed a case-control study to compare the prevalence and severity of subclinical CAD in RAO patients using CCTA. Patients with RAO showed higher CACS, and a higher prevalence of obstructive CAD compared to age- and gender-matched controls. In addition, significantly higher SIS and SSS in the RAO group suggest that subclinical CAD, which is more extensive with heavier plaque burden, was observed in RAO patients. RAO, especially CRAO, was an independent predictor for the presence of CAD with DS ≥ 50%, SIS > 4, and SSS > 8.
Non-arteritic RAO is often regarded as an ischemic stroke in the anterior territory of the central nervous system,21 and thus, our result should be correlated with that of cerebral ischemic stroke. Ischemic stroke is also known to have an overlap with CAD, and patients with ischemic stroke experience significant morbidity and mortality from CAD.22,23 There were several reports about the prevalence of subclinical CAD in patients with cerebral ischemic stroke. The prevalence of CAD was 62% and that of the obstructive CAD was 25.7% on coronary angiography.23 Using CCTA, the prevalence of obstructive CAD was 18% in patients with ischemic stroke or transient ischemic attack.22 In our study, RAO patients showed the prevalence of any CAD of 61% and obstructive CAD of 29% on CCTA, which is well matched with the prevalence in patients with ischemic stroke. This finding reaffirms that RAO shares the systemic vascular risk factors, including CAD with cerebral ischemic stroke.
It is also well-recognized that vascular risk factors are commonly observed in non-arteritic RAO patients.4,5,21,24 In our study, of the vascular risk factors common in both stroke and CAD, hypertension was the most common in RAO patients followed by dyslipidemia and diabetes.5,7,25,26,27,28 However, although RAO and CAD share such risk factors,5,7,25,26,27,28 the detailed etiology is deemed slightly different. The most common feasible etiology of RAO is embolism originating from large-artery atherosclerosis,7,24 and RAO and ischemic stroke are similar in temporal relevance and cause.5,6,24 On the other hand, CAD often developed due to coronary artery atherosclerosis itself and subsequent occlusion of the vessels.29
RAO and CAD are not closely related in terms of temporal aspect compared to ischemic strokes, according to Hong et al.24 who reported that, in the one-year vascular event rate after RAO, the ischemic stroke rate was 8.6% (ipsilesional, 6.6%; contralesional, 2.0%) and myocardial infarction was 0.7%. These anatomic, pathogenic, and temporal differences might not support the possible association between RAO and CAD. Although several studies have reported a prevalence of 3%–28% for CAD in RAO patients,2,7,30,31,32 those study results were limited by incomplete evaluation of CAD. Hayreh et al.2 reported that 26% of non-arteritic CRAO patients had ischemic heart disease, and there were significant differences when compared to the race-, age-, and period-matched population (P < 0.001). However, the investigators did not provide detailed information on how they defined ischemic heart disease, in either the patient group or the control population, and the association between RAO and ischemic heart disease was unclear. In addition, multiple risk factors, including diabetes and hypertension, were significantly different between RAO patients and controls in the study. In the EAGLE study, Callizo et al.7 analyzed 77 CRAO patients and reported that 6% had a previous history of CAD, and 16% were newly diagnosed as CAD with ECG and/or coronary angiography. However, the evaluation of CAD was not guided by the study protocol and was only initiated by ECG and echocardiographic findings at the time of RAO diagnosis. Not all the study participants underwent anatomic coronary evaluation. Therefore, the prevalence of subclinical CAD in RAO patients and the possible effects of the unadjusted risk factors remain unclear. In that respect, our study has value in that it could show the prevalence of subclinical CAD as well as the severity, extent, and plaque burden in comparison to controls.
CCTA has been widely used as a non-invasive imaging modality to evaluate CAD. Coronary artery calcium is a marker of coronary atherosclerosis and the CACS, which is used to quantify coronary artery calcification, is correlated with the coronary atherosclerotic burden and future adverse cardiovascular events. Therefore, CACS is very useful for refined risk stratification, especially in high-risk patients. To the best of our knowledge, we demonstrated for the first time higher CACS in RAO patients compared to control subjects suggesting the heavier plaque burden in patients with RAO. However, CACS alone does not provide information regarding the presence or absence of obstructive and non-obstructive CAD, whereas, CCTA, which provides direct visualization of coronary atherosclerosis, allows comprehensive evaluation regarding coronary anatomy, severity and extent of CAD, as well as plaque characteristics.33,34 The diagnostic accuracy and prognostic value of CCTA have largely been proved in symptomatic patients with low-intermediate risk.35,36,37,38 In addition to obstruction severity estimated by DS on CCTA, most well-known prognosticator, plaque extent and burden estimated by SIS and SSS provide important prognostic value.18,19
However, screening asymptomatic individuals is still widely debated, and CCTA is currently considered inappropriate in asymptomatic patients without known CAD and with low to intermediate risk for CAD.39 Although the value of detecting CAD using CCTA is still controversial, even in asymptomatic individuals with high risk, evidence supporting the use of CCTA in high-risk patients is accumulating.40 For example, in patients with ischemic stroke without chest pain, evaluation of the presence and extent of obstructive CAD by CCTA provided additional risk discrimination compared to using clinical risk factors and CACS.41 In our study, considering the common pathophysiology between RAO and ischemic stroke, CCTA was performed in patients with RAO. According to our CCTA findings, RAO patients showed more extensive and severe CAD than did the control groups, although they did not have any cardiac symptoms indicating CAD. CRAO in particular was an independent predictor for the presence of CAD with DS ≥ 50%, SIS > 4, and SSS > 8, which are known to be associated with increased risk for adverse cardiovascular events.12 Therefore, RAO patients may harbor increased future cardiovascular-related morbidity and mortality risk compared to control subjects. Of course, further study is needed to clarify whether cardiovascular risk stratification using CCTA in asymptomatic RAO patients is advantageous over traditional risk analysis.
Interestingly, among RAO subgroups, CRAO patients showed more severe and extensive CAPs on CCTA compared with their age- and gender-matched controls, while BRAO patients did not show any significant difference in CCTA findings compared to controls. Previously known risk factors of stroke and CAD, such as old age, men gender, and diabetes, were also related to subclinical CAD in our study.5,7,25,26,27,28 CRAO was also associated with obstructive CAD after adjusting for these risk factors, while BRAO was not an independent risk factor for obstructive CAD. These findings correspond to the previous finding that the risk of acute coronary syndrome was higher in the CRAO subgroup than that of BRAO.8 There may be differences in the pathogenic processes of arterial atherosclerosis between CRAO and BRAO. However, the number of BRAO patients included in this study was small, and further study with a larger number of patients is thus needed to clarify the association between BRAO and the risk of subclinical CAD.
Our study has some limitations. First is its retrospective design without a randomized control group. Although the selection of controls has been carefully considered regarding matching of age and gender, there is a possibility of unexpected confounding factors. In addition, our study was a cross-sectional study, and we could not confirm whether these results were associated with major cardiovascular events or mortality. Second, the subjects included were limited to the Korean population, and our results should thus be interpreted with consideration for ethnic differences. Lastly, only 41 RAO patients were included, which is not a fully sufficient number for a statistically significant evaluation. Differences between CRAO and BRAO, or other aspects of RAO such as visual and anatomical outcomes, might provide important clinical information. However, subgroup analyses were limited because of the small number of patients. RAO is a disease with a relatively low incidence, and the fact that CCTA is not a routine procedure for RAO, as well as the fact that it was performed only in patients who chose to undergo it, appears to have affected the number of patients. Our findings need to be replicated by larger prospective studies.
In conclusion, RAO patients have a higher prevalence of subclinical CAD and show more extensive and severe CAD on CCTA compared to the age- and gender-matched control subjects, suggesting that they may harbor future cardiovascular risk. Along with traditional vascular risk factors, RAO can be an additional and independent risk factor for CAD. RAO patients, particularly those with vascular risk factors, should undergo meticulous cardiovascular evaluations to prevent coronary artery-related morbidity and mortality.
Footnotes
Presentation: Paper presented at: The Association for Research in Vision and Ophthalmology (ARVO) annual meeting, April 2019, Vancouver, BC, Canada.
Funding: This study was supported by the National Research Foundation (NRF) grant 2016R1D1A1B03934724 and the NRF Bio & Medical Technology Development Program (Grant No. 2018M3A9B5021319) funded by the Korean government (MSIP and MSIT).
Disclosure: The authors have no potential conflicts of interest to disclose. The funding organization had no role in the design or conduct of this study.
- Conceptualization: Woo SJ, Yoon CH, Kim YK.
- Data curation: Kim YD, Kim YK, Yoon YE.
- Formal analysis: Kim YD, Kim YK, Yoon YE.
- Investigation: Kim YD, Kim YK, Yoon YE.
- Methodology: Woo SJ, Kim YD, Kim YK, Yoon YE.
- Validation: Woo SJ, Kim YK, Yoon YE.
- Writing - original draft: Kim YD, Kim YK, Yoon YE.
- Writing - review & editing: Woo SJ, Kim YD, Kim YK, Yoon YE, Yoon CH, Park KH.
SUPPLEMENTARY MATERIAL
Comparison of clinical characteristics and CCTA findings: RAO patients divided into BRAO and CRAO subgroups, and then compared with each corresponding age- and gender-matched control, respectively
References
- 1.Hayreh SS. Acute retinal arterial occlusive disorders. Prog Retin Eye Res. 2011;30(5):359–394. doi: 10.1016/j.preteyeres.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayreh SS, Podhajsky PA, Zimmerman MB. Retinal artery occlusion: associated systemic and ophthalmic abnormalities. Ophthalmology. 2009;116(10):1928–1936. doi: 10.1016/j.ophtha.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson LA, Warlow CP, Russell RW. Cardiovascular disease in patients with retinal arterial occlusion. Lancet. 1979;1(8111):292–294. doi: 10.1016/s0140-6736(79)90704-9. [DOI] [PubMed] [Google Scholar]
- 4.Chang YS, Jan RL, Weng SF, Wang JJ, Chio CC, Wei FT, et al. Retinal artery occlusion and the 3-year risk of stroke in Taiwan: a nationwide population-based study. Am J Ophthalmol. 2012;154(4):645–652.e1. doi: 10.1016/j.ajo.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 5.Klein R, Klein BE, Jensen SC, Moss SE, Meuer SM. Retinal emboli and stroke: the beaver dam eye study. Arch Ophthalmol. 1999;117(8):1063–1068. doi: 10.1001/archopht.117.8.1063. [DOI] [PubMed] [Google Scholar]
- 6.Park SJ, Choi NK, Yang BR, Park KH, Lee J, Jung SY, et al. Risk and risk periods for stroke and acute myocardial infarction in patients with central retinal artery occlusion. Ophthalmology. 2015;122(11):2336–2343.e2. doi: 10.1016/j.ophtha.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Callizo J, Feltgen N, Pantenburg S, Wolf A, Neubauer AS, Jurklies B, et al. Cardiovascular risk factors in central retinal artery occlusion: results of a prospective and standardized medical examination. Ophthalmology. 2015;122(9):1881–1888. doi: 10.1016/j.ophtha.2015.05.044. [DOI] [PubMed] [Google Scholar]
- 8.Chang YS, Chu CC, Weng SF, Chang C, Wang JJ, Jan RL. The risk of acute coronary syndrome after retinal artery occlusion: a population-based cohort study. Br J Ophthalmol. 2015;99(2):227–231. doi: 10.1136/bjophthalmol-2014-305451. [DOI] [PubMed] [Google Scholar]
- 9.Youn TS, Lavin P, Patrylo M, Schindler J, Kirshner H, Greer DM, et al. Current treatment of central retinal artery occlusion: a national survey. J Neurol. 2018;265(2):330–335. doi: 10.1007/s00415-017-8702-x. [DOI] [PubMed] [Google Scholar]
- 10.Abdulla J, Abildstrom SZ, Gotzsche O, Christensen E, Kober L, Torp-Pedersen C. 64-multislice detector computed tomography coronary angiography as potential alternative to conventional coronary angiography: a systematic review and meta-analysis. Eur Heart J. 2007;28(24):3042–3050. doi: 10.1093/eurheartj/ehm466. [DOI] [PubMed] [Google Scholar]
- 11.Hulten EA, Carbonaro S, Petrillo SP, Mitchell JD, Villines TC. Prognostic value of cardiac computed tomography angiography: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;57(10):1237–1247. doi: 10.1016/j.jacc.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Bittencourt MS, Hulten E, Ghoshhajra B, O'Leary D, Christman MP, Montana P, et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging. 2014;7(2):282–291. doi: 10.1161/CIRCIMAGING.113.001047. [DOI] [PubMed] [Google Scholar]
- 13.Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O'Gara P, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010;56(22):1864–1894. doi: 10.1016/j.jacc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Hur J, Choi BW. Cardiac CT imaging for ischemic stroke: current and evolving clinical applications. Radiology. 2017;283(1):14–28. doi: 10.1148/radiol.2016152043. [DOI] [PubMed] [Google Scholar]
- 15.Lee H, Yoon YE, Park JB, Kim HL, Park HE, Lee SP, et al. The incremental prognostic value of cardiac computed tomography in comparison with single-photon emission computed tomography in patients with suspected coronary artery disease. PLoS One. 2016;11(8):e0160188. doi: 10.1371/journal.pone.0160188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 17.Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51(4) Suppl:5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 18.Al-Mallah MH, Qureshi W, Lin FY, Achenbach S, Berman DS, Budoff MJ, et al. Does coronary CT angiography improve risk stratification over coronary calcium scoring in symptomatic patients with suspected coronary artery disease? Results from the prospective multicenter international CONFIRM registry. Eur Heart J Cardiovasc Imaging. 2014;15(3):267–274. doi: 10.1093/ehjci/jet148. [DOI] [PubMed] [Google Scholar]
- 19.Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50(12):1161–1170. doi: 10.1016/j.jacc.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 20.Leber AW, Becker A, Knez A, von Ziegler F, Sirol M, Nikolaou K, et al. Accuracy of 64-slice computed tomography to classify and quantify plaque volumes in the proximal coronary system: a comparative study using intravascular ultrasound. J Am Coll Cardiol. 2006;47(3):672–677. doi: 10.1016/j.jacc.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 21.Biousse V, Nahab F, Newman NJ. Management of acute retinal ischemia: follow the guidelines! Ophthalmology. 2018;125(10):1597–1607. doi: 10.1016/j.ophtha.2018.03.054. [DOI] [PubMed] [Google Scholar]
- 22.Calvet D, Touzé E, Varenne O, Sablayrolles JL, Weber S, Mas JL. Prevalence of asymptomatic coronary artery disease in ischemic stroke patients: the PRECORIS study. Circulation. 2010;121(14):1623–1629. doi: 10.1161/CIRCULATIONAHA.109.906958. [DOI] [PubMed] [Google Scholar]
- 23.Amarenco P, Lavallée PC, Labreuche J, Ducrocq G, Juliard JM, Feldman L, et al. Prevalence of coronary atherosclerosis in patients with cerebral infarction. Stroke. 2011;42(1):22–29. doi: 10.1161/STROKEAHA.110.584086. [DOI] [PubMed] [Google Scholar]
- 24.Hong JH, Sohn SI, Kwak J, Yoo J, Ahn SJ, Woo SJ, et al. Retinal artery occlusion and associated recurrent vascular risk with underlying etiologies. PLoS One. 2017;12(6):e0177663. doi: 10.1371/journal.pone.0177663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Babikian V, Wijman CA, Koleini B, Malik SN, Goyal N, Matjucha IC. Retinal ischemia and embolism. Etiologies and outcomes based on a prospective study. Cerebrovasc Dis. 2001;12(2):108–113. doi: 10.1159/000047689. [DOI] [PubMed] [Google Scholar]
- 26.Franco OH, Steyerberg EW, Hu FB, Mackenbach J, Nusselder W. Associations of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med. 2007;167(11):1145–1151. doi: 10.1001/archinte.167.11.1145. [DOI] [PubMed] [Google Scholar]
- 27.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004;328(7455):1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 29.Crea F, Libby P. Acute coronary syndromes: the way forward from mechanisms to precision treatment. Circulation. 2017;136(12):1155–1166. doi: 10.1161/CIRCULATIONAHA.117.029870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hankey GJ, Slattery JM, Warlow CP. Prognosis and prognostic factors of retinal infarction: a prospective cohort study. BMJ. 1991;302(6775):499–504. doi: 10.1136/bmj.302.6775.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leisser C. Are there differences between internal carotid artery and aortic arch plaques among patients with retinal artery occlusion and anterior ischaemic optic neuropathy? Klin Monatsbl Augenheilkd. 2014;231(11):1084–1087. doi: 10.1055/s-0034-1368574. [DOI] [PubMed] [Google Scholar]
- 32.Callizo J, Feltgen N, Ammermann A, Ganser J, Bemme S, Bertelmann T, et al. Atrial fibrillation in retinal vascular occlusion disease and non-arteritic anterior ischemic optic neuropathy. PLoS One. 2017;12(8):e0181766. doi: 10.1371/journal.pone.0181766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52(21):1724–1732. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 34.Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359(22):2324–2336. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 35.Guaricci AI, Pontone G, Brunetti ND, De Rosa F, Montrone D, Guglielmo M, et al. The presence of remodeled and mixed atherosclerotic plaques at coronary CT angiography predicts major cardiac adverse events - The CAFÉ-PIE Study. Int J Cardiol. 2016;215:325–331. doi: 10.1016/j.ijcard.2016.04.129. [DOI] [PubMed] [Google Scholar]
- 36.Maffei E, Seitun S, Martini C, Aldrovandi A, Cervellin G, Tedeschi C, et al. Prognostic value of computed tomography coronary angiography in patients with chest pain of suspected cardiac origin. Radiol Med. 2011;116(5):690–705. doi: 10.1007/s11547-011-0647-z. [DOI] [PubMed] [Google Scholar]
- 37.SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385(9985):2383–2391. doi: 10.1016/S0140-6736(15)60291-4. [DOI] [PubMed] [Google Scholar]
- 38.Maffei E, Midiri M, Russo V, Rengo M, Tedeschi C, Spagnolo P, et al. Rationale, design and methods of CTCA-PRORECAD (Computed Tomography Coronary Angiography Prognostic Registry for Coronary Artery Disease): a multicentre and multivendor registry. Radiol Med. 2013;118(4):591–607. doi: 10.1007/s11547-012-0912-9. [DOI] [PubMed] [Google Scholar]
- 39.Cho I, Al'Aref SJ, Berger A, Ó Hartaigh B, Gransar H, Valenti V, et al. Prognostic value of coronary computed tomographic angiography findings in asymptomatic individuals: a 6-year follow-up from the prospective multicentre international CONFIRM study. Eur Heart J. 2018;39(11):934–941. doi: 10.1093/eurheartj/ehx774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Auer J. Coronary evaluation in patients with stroke: recognizing the risk. Atherosclerosis. 2015;238(2):427–429. doi: 10.1016/j.atherosclerosis.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 41.Hur J, Lee KH, Hong SR, Suh YJ, Hong YJ, Lee HJ, et al. Prognostic value of coronary computed tomography angiography in stroke patients. Atherosclerosis. 2015;238(2):271–277. doi: 10.1016/j.atherosclerosis.2014.10.102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of clinical characteristics and CCTA findings: RAO patients divided into BRAO and CRAO subgroups, and then compared with each corresponding age- and gender-matched control, respectively