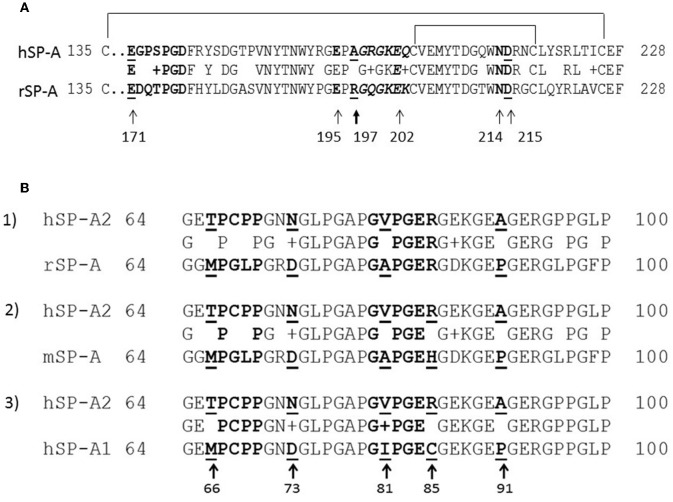
Figure 1.
Partial sequence alignment of the CRD (A) and CDM (B) domains of rodent and human SP-A. (A) Alignment of the CRD domains of human SP-A2 1A0 variant (hSP-A2) and rat SP-A carbohydrate recognition domain. Amino acid numbering is based on the mature peptide of rat SP-A. Arrows point to Ca++-coordination residues. (B) Alignment of the collagen-like domains of human SP-A1 (6A2) (hSP-A1) and hSP-A2 1A0 variant, mouse SP-A (mSP-A), and rat SP-A (rSP-A). All alignments are made to hSP-A2 (1A0). The aligned sequences shown encompass the kink peptide (bolded residues 66–50) to the end of the CDM. Arrows point to core amino acids in the CDM that distinguish SP-A1 and SP-A2; the numbering is based on the precursor molecule. Residues shown in italicized red font show the sequence of embedded integrin binding motifs in the CDM. Sequence alignments were performed using ClustalW.