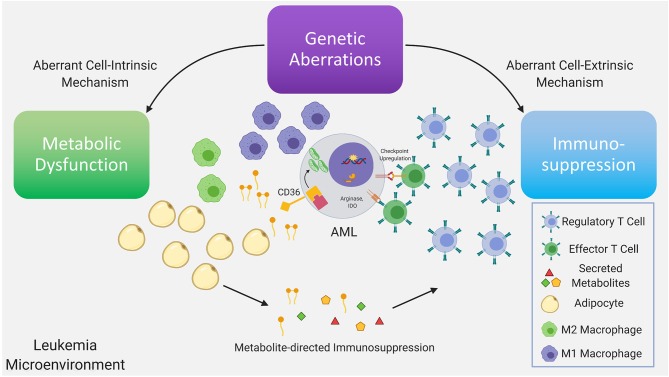
Figure 1.
Genetic aberrations drive dysfunctional cell-intrinsic and -extrinsic signaling, with the former having direct metabolic consequences and the latter having direct immunologic consequences. Further dysregulation is driven via interactions between the altered metabolome and immune system as a consequence of the existing immunometabolic network. Recurrent genetic mutations found in AML have been demonstrated to promote T regulatory cell expansion, suppress proliferation of T effector cells, skew macrophage maturation toward the suppressive M2 phenotype. A subset of these immune suppressive mechanisms are mediated by metabolites. In AML, depletion of amino acids, such as tryptophan and asparagine via increased arginase-1 and IDO levels in leukemia cells, M2-macrophages and MDSC, limits T effector activity. Furthermore, mobilization of fatty acids in the leukemia microenvironment from adipocytes, a subset of which can function as pro-inflammatory lipid mediators, may simultaneously provide anti-apoptotic and immunosuppressive signals inhibiting T effector activity and promoting suppressive immune cell types. In leukemia cells the metabolic regulatory network that is stimulated by adipocytes may include upregulation of the lipid scavenger receptor CD36, the fatty acid activated receptor/transcription factor PPARG, the fatty acid binding protein (FABP4) along with BCL2. Upstream genetic determinants/mutations regulating specific metabolic adaptations in leukemia cells is the subject of ongoing research.