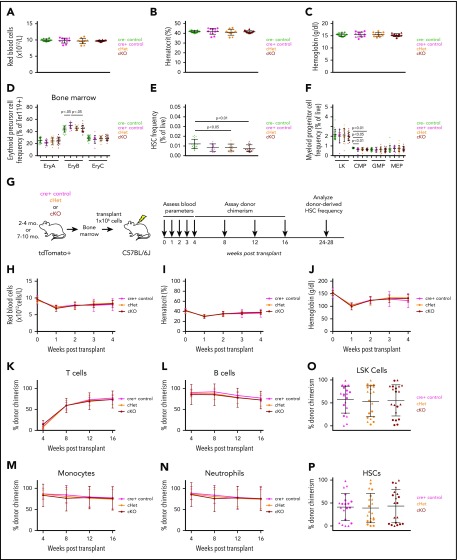
Figure 2.
Hematopoietic cell–specific Gdf11 deletion does not alter adult erythropoiesis, RBC parameters, or HSPC frequency or hematopoiesis following adult BM transplantation. (A) RBC counts, (B) hematocrit, and (C) hemoglobin levels in 2- to 4-month-old mice (n = 10-12 mice per genotype; males and females pooled). (D-F) Frequency of BM (D) EPCs, (E) HSCs, and (F) myeloid progenitor cells within 2- to 4-month-old mice (n = 10-12 mice per genotype; males and females pooled). (G) Experimental design. BM was harvested from 2- to 4-month-old or 7- to 10-month-old Gdf11 cKO, cHet, and cre+ control mice. Cells from sex- and genotype-matched donors were pooled and 1 × 106 BM cells were injected IV into lethally irradiated sex-matched C57BL/6J recipients. Recipients were monitored for blood parameters and donor cell chimerism as indicated. (H) RBC counts, (I) hematocrit, and (J) hemoglobin levels within recipient mice at 0 (baseline), 1, 2, 3, and 4 weeks posttransplant (n = 16-20 recipients per donor genotype; males and females pooled). (K-N) tdTomato+ donor-derived peripheral blood cell chimerism levels determined monthly for (K) CD3+ T cells, (L) B220+ B cells, (M) CD11b+ Ly6G− monocytes, and (N) CD11b+ Ly6G+ neutrophils in transplant recipients (n = 16-20 recipients per donor genotype; males and females pooled). (O-P) tdTomato+ donor-derived BM cell chimerism levels for BM (O) Lin−Sca-1+c-Kit+ and (P) Lin−Sca-1+c-Kit+CD48−CD150+ cells in transplant recipients (n = 16-20 recipients per donor genotype; males and females pooled). Data are plotted as (H-N) mean ± SD or as (A-F, O-P) individual data points overlaid with mean ± SD. Statistics for panels H-N were calculated using a repeated-measures ANOVA. Statistics for panels A-F, O-P were calculated using a 1-way ANOVA with Bonferroni posttest correction. For all panels, no differences with P < .05 were detected. (A-F; O-P) Circles: males; triangles: females.