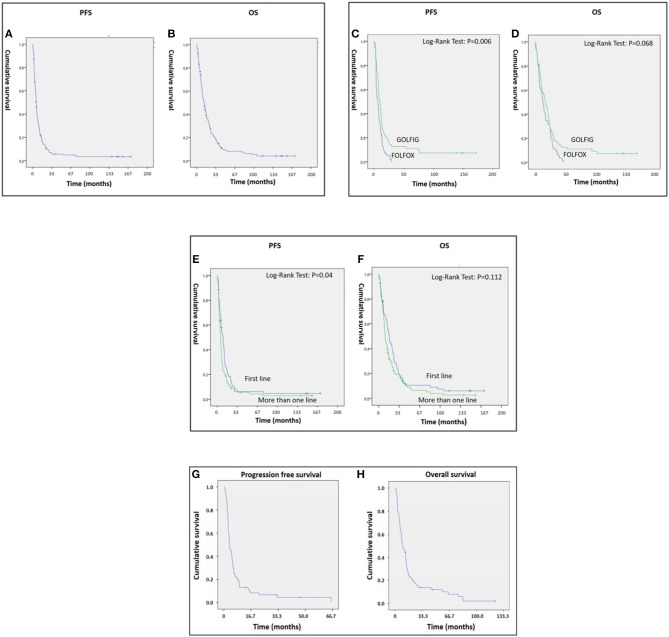
Figure 1.
(A–H) Panels A and B represent PFS (A) and OS (B) of metastatic colorectal cancer (mCRC) patients who received the GOLFIG (gemcitabine, oxaliplatin, LF, and fluorouracil poly-chemotherapy with granulocyte-macrophage colony stimulating factor and interleukin-2) regimen and enrolled in the GOLFIG-1 trial, GOLFIG-2 trial, and real-world treatment. (C,D) represent the updated results of the GOLFIG-2 trial including 124 mCRC patients randomized to receive frontline treatment with GOLFIG regimen (62 pts) or FOLFOX (fluoropyrimidine backbone coupled to oxaliplatin) chemotherapy (62 pts). GOLFIG showed superiority over FOLFOX in terms of PFS (C) and a trend to superiority in terms of OS (D). All of the patients were allowed a free second line with or without anti–epidermal growth factor receptor (EGFR) or vascular-endothelial growth factor (VEGF) monoclonal antibodies (mABs). (E,F) represents the PFS (E) and OS (F) of mCRC patients who received GOLFIG chemo-immunotherapy as frontline (62 pts enrolled in the GOLFIG-2 trial) or second/third line (117 patients, of whom 49 enrolled in the GOLFIG-1 trial and the remaining as real-world treatment). (G,H) represent PFS (G) and OS (H) of the real-life subset of patients.