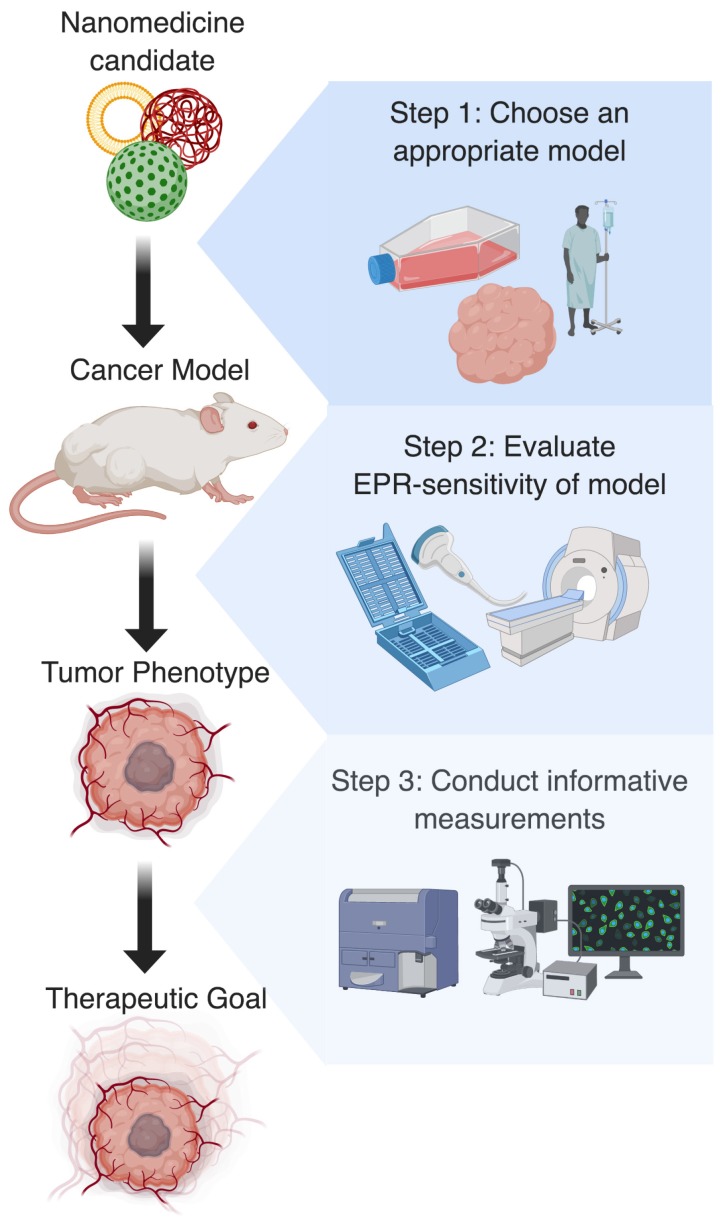
Figure 4.
Ideal preclinical workflow for research using EPR-adaptive delivery strategies. Step 1: Based on the clinical target and nanomedicine formulation, the most appropriate tumor model should be identified. Step 2: Following model development, the EPR-sensitivity of the tumor should be assessed using imaging or histology, as this is useful for predicting the magnitude of the expected delivery improvement. Step 3: Finally, a procedure for measuring uptake or therapeutic benefit should be chosen that highlights the spatial or temporal improvement conferred by the delivery strategy. Together, these actions should create more interpretable and generalizable results that will aid in the clinical translation of discoveries.