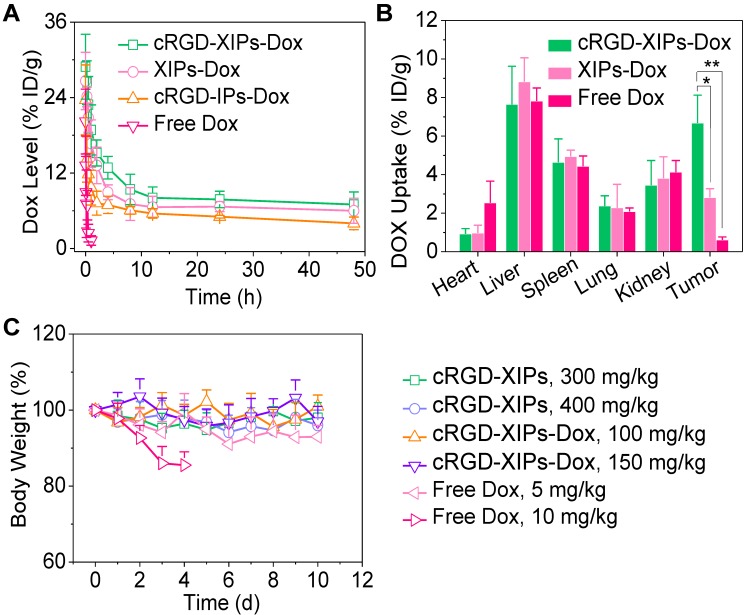
Figure 3.
In vivo pharmacokinetics, biodistribution and toleration studies of cRGD-XIPs-Dox in mice (n = 3). (A) Pharmacokinetics of cRGD-XIPs-Dox in healthy C57BL/6 mice (10 mg Dox/kg). XIPs-Dox (non-targeted), cRGD-IPs-Dox (non-crosslinked) and free Dox were used as controls. (B) Biodistribution of free Dox and Dox delivered by cRGD-XIPs or XIPs in C57BL/6 mice bearing B16 tumor 4 h post iv injection. (C) Toleration studies of tumor-free C57BL/6 mice towards cRGD-XIPs, cRGD-XIPs-Dox and free Dox. *p< 0.05 and **p < 0.01 (one-way Anova and Tukey multiple comparisons tests).