Summary
In order to reset the immune system to baseline function, autologous hematopoietic stem cell transplantation (HSCT) has been performed in patients with multiple sclerosis (MS). After June 2015, 617 new consecutive patients with MS were autografted in our center with non‐frozen peripheral blood stem cells. The autografts were performed on an out‐patient basis, after conditioning with cyclophosphamide and rituximab. The aim of the study was the assessment of both safety and efficacy of the method. The study’s primary co‐end‐points were recovery of granulocyte and platelet counts and transplant‐related mortality. Secondary end‐points were overall survival and clinical response (improvement or stabilization of the self‐reported expanded disability status scale score). The protocol was registered in ClinicalTrials.gov identifier NCT02674217.0. We included 401 females and 216 males, with a median age of 46 years. A total of 259 patients had relapsing–remitting MS (RRMS), 228 had secondary progressive (SPMS) and 130 had primary progressive (PPMS) multiple sclerosis. All procedures were initially performed on an out‐patient basis and only 32 individuals (5%) required hospitalization. One to three aphereses (median 1) were required to harvest at least 1 × 106/kg viable CD34+ cells. The total number of viable CD34+ infused cells ranged between 1 and 37·83 × 106/kg (median 5·68). Patients recovered more than 0·5 × 109/l absolute granulocytes by day 8 (median, range = 2–14), and platelet values were above 20 × 109/l by day 4 (median, range = 0–11). Eleven individuals required red blood cells and six needed platelet transfusions. To date, there have been no deaths attributable to the transplant, yielding a 30‐month overall survival of 100%. Patients have been followed for 3–42 months (median = 12). The overall response rate (decrease or stabilization of the self‐reported EDSS score) at 12 months was 78% for all patients (83% in RRMS, 78% in PPMS and 73% in SPMS), while the disability progression‐free survival was 82% for all patients (86% in RRMS, 78·5% in SPMS and 78% in SPMS). Changes in the self‐reported EDSS score in parallel with neurological improvement were observed in people with all types of MS after HSCT, employing the ‘Mexican method’.
Keywords: autoimmunity, multiple sclerosis, stem cells, transplantation
Changes in the self‐reported EDSS score accordant with neurological improvement, were observed in peoples with all types of MS after HSCT, employing the ‘Mexican method’.
![]()
Introduction
Multiple sclerosis (MS) is a chronic neurological inflammatory disease resulting from the immune‐mediated injury and destruction of central nervous system myelin, and concomitant variable axonal injury 1. In the 1990s, various studies in animal models suggested that hematopoietic stem cell transplantation (HSCT) could be a viable option in the management of autoimmune diseases; further, some patients responded clinically, which led to the theory that high‐dose chemotherapy followed by HSCT rescue could ‘reset’ the immune system by controlling or depleting autoreactive clones, and conditioning immunological tolerance after immune reconstitution 2; this led to the conclusion that HSCT could be a feasible therapeutic recourse in the management of MS 2, 3, 4, 5, 6, 7.
Autologous HSCTs have been administered to patients with MS for more than 20 years; worldwide, more than 2000 HSCTs have been performed 2, 3, 4, 5, 6, 7, 8, with considerably more promising neurological outcomes in patients with relapsing–remitting MS (RRMS) and/or in those with an inflammatory pattern by magnetic resonance imaging (MRI) during pretransplant screening. Transplant‐related mortality (TRM) in MS managed with HSCT was formerly considered a limiting factor, but has decreased to less than 2% 2, 3, 4, 5, 6, 7, 8, probably as a result of the less toxic conditioning regimens currently administered. Recent data obtained from over 1000 autologous transplants performed worldwide to manage MS have yielded an overall survival greater than 90% at 5 years post‐transplant and a progression‐free survival of approximately 50%; the most frequent cause of morbidity and mortality has been autoimmune disease recurrence 2, 3, 4, 5, 6, 7, 8.
Since 1993 we have managed patients with HSCTs using novel methods to decrease the procedure’s toxicity and the patients’ expenses 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19; to date, more than 1000 HSCTs have been performed at our center for the management of various diseases, including acute leukemia, chronic leukemia, aplastic anemia, myeloma, lymphoma, myelodysplasia and autoimmune diseases, including MS 19. The previously reported characteristics of our HSCT method include the administration of cells on an out‐patient basis, the collection of stem cells from peripheral blood and the avoidance of cellular freezing and thawing, to both increase the viability of hematopoietic cells in the graft and decrease costs 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19. These modifications to traditional stem cell transplantation methods have made the procedure affordable to patients in socio‐economically impoverished conditions, a characteristic of the population in developing countries 19. Our previous experience autografting hematological malignancies 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 led us to develop a unique program, grafting non‐cryopreserved autologous hematopoietic stem cells in patients with MS, using a modified autografting conditioning regimen. We report herein the results of engrafting, toxicity and neurological course in a group of 617 patients with MS who were autografted following the ‘Mexican method’ of conditioning, based on cyclophosphamide (Cy) and rituximab 7, 8; the aim of the study is the assessment of both the safety and efficacy of the method.
Material and methods
Patients
All consecutive patients with MS referred to our center for a HSCT between June 2015 and March 2019 were prospectively included into the study. RRMS, secondary progressive (SPMS) and primary progressive (PPMS) cases of multiple sclerosis were considered suitable candidates if their Karnofsky performance status 21 was greater than 70% and they had an expanded disability status scale (EDSS) score 1 of 8 or below, 2 weeks prior to transplantation. None of the patients had received myelotoxic agents prior to inclusion into the study and all had a normal complete blood cell count when cellular mobilization was begun. All patients underwent a washout period of at least 3 months of other immunosuppressive agents. The pretransplant EDSS score assessment was performed by the same neurologist.
Study aims
The study’s primary co‐end‐points were the recovery of granulocyte and platelet counts and TRM. Secondary endpoints were overall survival (OS) and clinical response (improvement or stabilization of the self‐reported EDSS score). OS was defined as the time elapsed between the HSCT and death from any cause, registering all patients who were alive on their last follow‐up date. All procedures in this study were conducted in accordance with the Ethics Committee of the Clínica Ruiz (Conbioetica 21CEI00120130605, Registry no. 13 CEI 21 114 126). All patients signed a consent form after being fully informed on the procedure’s benefits and potential complications. Subjects were instructed to provide information on their neurological status and adverse events every 3 months post‐transplant, in special forms 22 submitted by e‐mail. Other variables, such as visual field defects or deep sensory examinations, were not used when assessing the response. Differences in the reported EDSS scores were analyzed with Fisher’s exact test (P < 0·05). The protocol is registered in ClinicalTrials.gov, identifier NCT02674217.
Peripheral blood stem cell (PBSC) mobilization and apheresis
PBSC were mobilized with cyclophosphamide (Cy) and filgrastim [granulocyte colony‐stimulating factor (G‐CSF)]. Intravenous Cy (50 mg/kg) was administered over a 120‐min period on days –11 and –10. Subcutaneous G‐CSF (10 μg/kg/bid) was applied on days –9 to –1. Using either a peripheral vein or a Mahurkar‐type subclavian catheter, the apheresis procedure was performed on day –2, using an Amicus (Fresenius Kabi, Deerfield, IL, USA) or Spectra Optia machine (Terumo BCT, Lakewood, CO, USA), following the Spin‐Nebraska protocol 23. The aim of the apheresis was to obtain at least 1 × 106 viable CD34+ cells/kg. CD34+ cells in peripheral blood were not measured prior to the apheresis procedures.
Conditioning and autografting
After collecting the required number of peripheral CD34+ blood cells, intravenous Cy (50 mg/kg) was administered over a 120‐min period on days –2 and –1, followed by 2‐mercaptoethane sulfonate Na (MESNA) (1000 mg/m2 over a 180‐min period), ondansetron 8 mg, dexamethasone 4 mg and pantoprazole 40 mg, all on an out‐patient basis. Figure 1 summarizes these data. After the intravenous Cy, ondansetron (4 mg every 12 h after chemotherapy), oral cotrimoxazole (800/160 mg every 24 h), oral fluconazole (200 mg) and oral acyclovir (400 mg every 12 h) were administered to all patients until granulocytes increased above 0·5 × 109/l; throughout this period, a complete clinical examination and full laboratory work‐up were performed in all patients every 48 h. After granulocyte recovery, rituximab was administered over a 3‐h period. In an effort to prevent infections and MS relapses in the subsequent 6 months, we prescribed cotrimoxazole 800/160 mg bid three times a week, acyclovir 800 mg daily and rituximab (100 mg) every 2 months over a 12‐month period. The cumulative dose of Cy was 200 mg/kg. The decision to receive rituximab therapy, despite known John Cunningham virus antigenemia, was made by each individual patient on the informed consent form. The first 63 subjects prospectively accrued in the study, received a dose of rituximab (375 mg/m2 over a 3 h period) once the granulocyte count recovered, followed by rituximab, 100 mg every 2 months for 1 year, while subsequent patients received a single high dose of rituximab (1000 mg, fixed dose) after granulocyte recovery.
Figure 1.
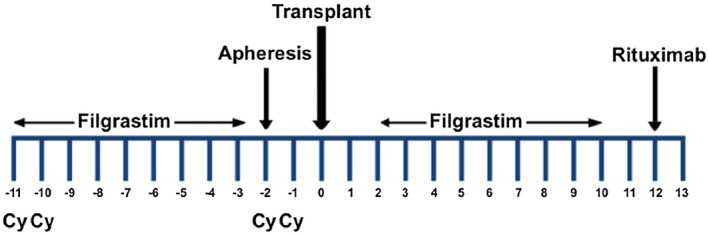
Schema of the ‘Mexican method’ for autologous hematopoietic stem cell transplantation in multiple sclerosis. Cy = cyclophosphamide, 50 mg/kg.
Apheresis product preservation, studies and infusion
The products of apheresis were stored in 1‐ml aliquots with ACD‐A (Baxter Healthcare, Deerfield IL, USA) at 4°C, in 1000‐ml transfer packs made of impermeable gas and polyvinyl chloride plastic film, for up to 96 h. Total white mononuclear cells (MNC) and CD34‐positive cell counts were obtained by flow cytometry 24 in an EPICS Gallios system (Coulter Electronics, Hialeah, FL, USA), with a phycoerythrin‐labeled anti‐CD34 HPCA‐2 monoclonal antibody (Becton Dickinson, San José, CA, USA) and a fluorescein isothiocyanate‐tagged anti‐CD45 monoclonal antibody (Beckman Coulter, Hialeah, FL, USA), gating in 7¢‐amino‐actinomycin‐D‐excluding cells. Stored MNC viability studies were conducted by propidium iodide exclusion in the flow cytometer. The apheresis products obtained on days –2 and –1 were reinfused into the patients on days 0 and +1 respectively, after storage in a conventional blood bank refrigerator (Thermoforma, Marietta OH, USA).
Results
Patients
Six hundred and seventeen subjects were enrolled into the study after June 2015. Two hundred and sixteen were male (35%), 401 were female (65%) and their median age was 46 years (range = 18–73). One hundred and thirty (130) subjects (21%) had PPMS, 259 (42%) had RRMS and 228 (37%) had SPMS. Their median EDSS score was 5·5 (range = 0–8) with an interquartile range (IQR) of 4–6·5. All the autografts were performed on an out‐patient basis and only 32 individuals required hospitalization during the procedure: 15 as a result of neutropenic fever, six due to a MS flare, four required placement of a chest tube to resolve a pneumothorax, three had persistent nausea and/or vomiting and one each developed a cardiac arrhythmia, rectal bleeding, urinary tract infection and a minimal pleural effusion. All hospitalizations lasted a maximum of 48 h.
In order to obtain a minimum of 1× 106 viable CD34+ cells/kg, one to three aphereses were required (median 1). The total number of infused viable CD34+ cells ranged between 1 and 37·83 × 106/kg (median 5·68 × 106/kg). A single apheresis was sufficient to collect at least 1 × 106/kg CD34+ cells in 94% of individuals. Patients recovered more than 0·5 × 109/l absolute granulocytes by day 8 (median, range = 2–14) and platelet values were above 20 × 109/l by day 4 (median, range = 0–11). Eleven individuals required red blood cells and six needed platelet transfusions. To date, there have been no deaths attributable to the transplant, and our 30‐month overall patient survival is 100%. Patients have been followed for 3–42 months (median = 12). No opportunistic infections have been recorded. Low‐dose, prophylactic rituximab was administered to 63 (10.2%) of the 617 patients over a 1‐year period.
Follow‐up
Four hundred and forty‐four of the 617 patients (72%) have been followed for 3 or more months (range = 3–42), and have completed the electronically provided formats. Not all patients answered the format, so follow‐up compliance was 71% after 3 or more months (240 patients at 1 year, 136 at 2 years and 19 at 3 years). Of these, 113 (47%) reported improvement in the EDSS score and 75 (31%) reported stabilization; accordingly, the response rate (RR = improvement + stabilization) was 188/240 = 78%. The overall RR was 83% in RRMS, 78% in PPMS and 73% in SPMS patients. In the whole group, the EDSS was assessed 3, 6, 9, 12 and 15 months after the graft, and decreased from an initial mean of 5·1 points to a mean of 4·5 points [P = 0·0002, 95% confidence interval (CI)]; see Fig. 2. Figures 3, 4 and 5 are the waterfall plots of the EDSS score changes 12 months post‐transplant. The RR at 12 months was 17% in RRMS, 22% in PPMS and 27% in SPMS patients (Fig. 6). No magnetic resonance imaging studies were systematically obtained to assess patient response.
Figure 2.
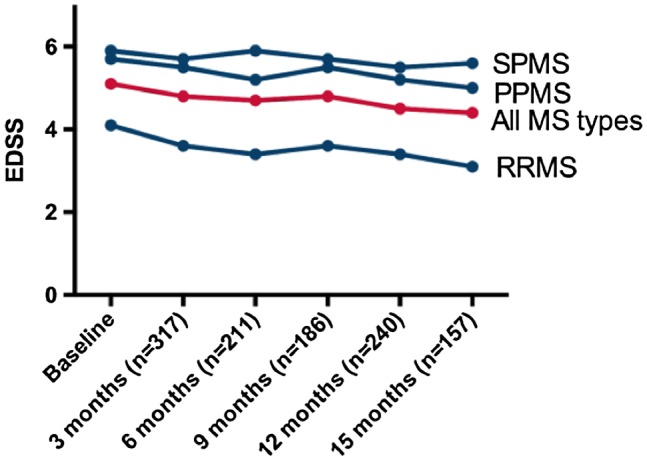
Changes in the expanded disability status scale (EDSS) score after hematopoietic stem cell transplantation (HSCT) in patients with secondary progressive (SPMS), primary progressive (PPMS) and relapsing–remitting multiple sclerosis (RRMS). The EDSS decrease is most marked in RRMS.
Figure 3.
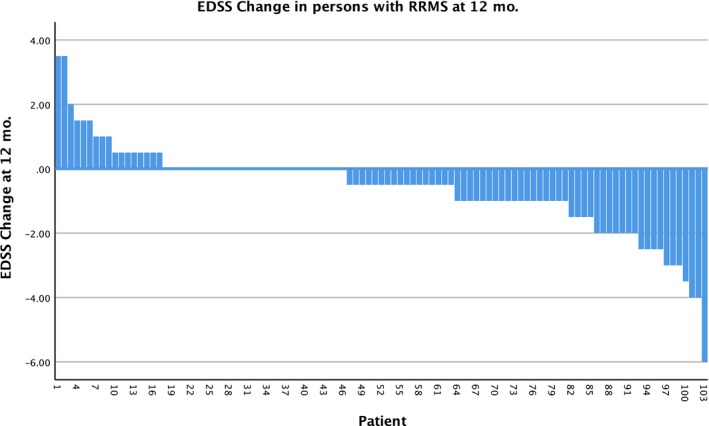
Waterfall plot of the changes in the expanded disability status scale (EDSS) score 12 months after hematopoietic stem cell transplantation (HSCT) in patients with relapsing–remitting multiple sclerosis (RRMS), n = 103. The overall response rate was 83%.
Figure 4.
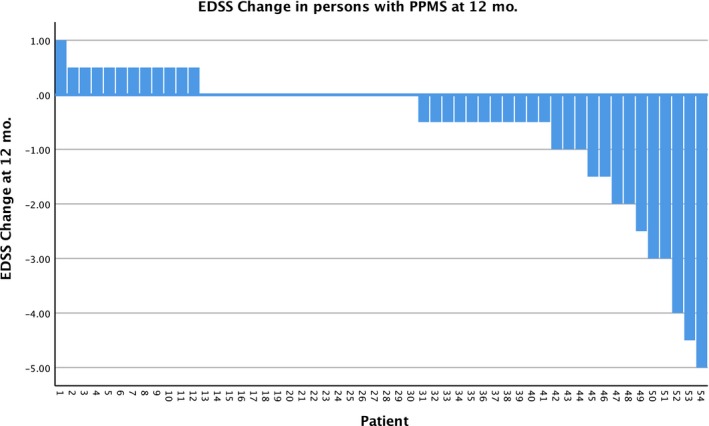
Waterfall plot of the changes in the expanded disability status scale (EDSS) score 12 months after hematopoietic stem cell transplantation (HSCT) in patients with primary progressive multiple sclerosis (PPMS), n = 54. The overall response rate was 78%.
Figure 5.
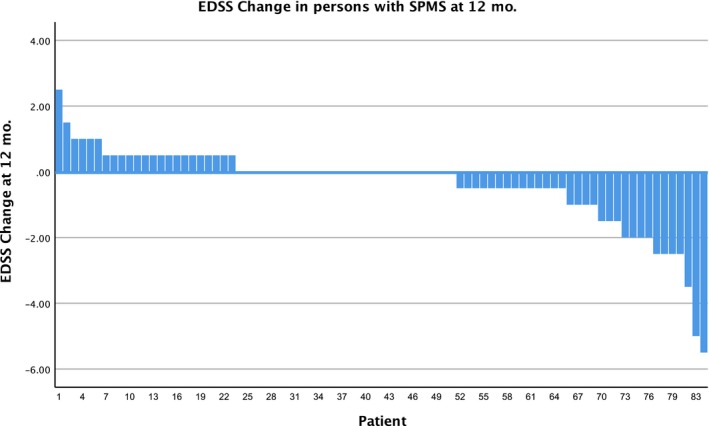
Waterfall plot of the changes in the expanded disability status scale (EDSS) score 12 months after hematopoietic stem cell transplantation (HSCT) in patients with secondary progressive (SPMS), n = 83. The overall response rate was 73%.
Figure 6.
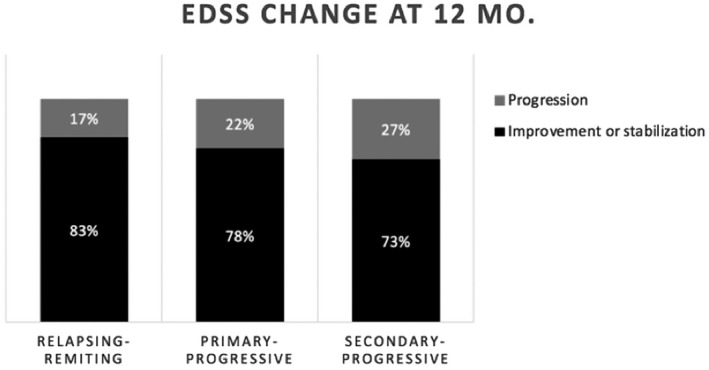
Overall response rate [stabilization or improvement in the expanded disability status scale (EDSS) score] at 12 months in patients with different types of multiple sclerosis.
Discussion
Although still not considered standard therapy in the management of some forms of MS, autologous HSCT has been attempted with promising results 1, 2, 3, 4, 5, 6, 7, 8. The mortality rate of autologous HSCT was a solid argument precluding it as a viable therapeutic option in various diseases, but as both morbidity and mortality rates have substantially decreased in recent years as a result of modifications to the procedure, it appears to be gaining acceptance in the medical milieu. In hematological malignancies, we and others have shown that the mortality rate of the autografts can be substantially decreased to figures below 5% 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 if the transplant is performed with PBSC on an out‐patient basis, and using non‐frozen, non‐thawed stem cells 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20. After 25 years of autografting more than 1000 patients with hematological malignancies 16, 17, 20, 26, we have now grafted patients with MS 7, 8 using a novel low‐intensity conditioning regimen, unlike that commonly recommended in other parts of the world. A key point in our protocol is the administration of cyclophosphamide for 2 days – 7 days apart – instead of on 4 consecutive days. The rationale hinges on three points: 1, 8: to mobilize cells with cyclophosphamide for the autograft 2, 8 to allow the use of a refrigerated rather than a frozen autograft, and to decrease toxicity 8, 26, 27. The rapid bone marrow recovery we have observed using refrigerated blood cell grafts is concordant with our experience in individuals with plasma cell myeloma and lymphomas who received high‐dose therapy and an autotransplant 25. In the literature, there are data on the use of unsplit cyclophosphamide 200 mg/kg without stem cell support 27: 43% of patients treated at Johns Hopkins had neutropenic fever, required a median of two platelet and two packed red cell transfusions, their neutrophil recovery was delayed (13 days) and 2% developed severe hemorrhagic cystitis; these complications have not been observed following our method.
The major drawback to our study is the lack of complete follow‐up of all the autografted patients’ neurological course; further, most EDSS scores were self‐reported either by the patient or occasionally by their physician, leading to reference bias when assessing their responses. Interestingly, some studies have reported good consistency when correlating self‐reported EDSS scores with physician‐assessed values 28, 29. Our finding of an overall RR (either stabilization or improvement in the neurological condition) of 78% in all forms of MS is interesting, and similar to what has been published in other centers 2, 3, 5, 30, 31, 32, 33, 34, 35; as expected, the overall RR varies in accordance with the type of MS: 83% in RRMS, 78% in PPMS and 73% in SPMS. These figures support our decision to graft individuals with all types of MS. Autografting yields favorable responses in 70–80% of patients in different parts of the world, while standard and novel drugs improve the patient’s clinical course and wellbeing in, at most, 50% of cases 30, 31, 32, 33, 34, 35. The neurological response in MS can also be evaluated with the patient’s ‘disability progression’ which, in patients with an EDSS < 5·5, is defined as an increase of > 1·0, and in patients with an EDSS > 5·5 it reflects an increase > 0·5 34. With this tool, the disability progression‐free survival in our group was 82% in all patients (86% in RRMS, 78·5% in SPMS and 78% in SPMS).
Additional studies are needed to both confirm the feasibility of the autografting method in patients with MS, as well as to assess the efficacy of the procedure and compare the results of auto‐HSCT with those obtained with other forms of immunosuppression 23, 29, 30, 31, 32, 33.
Disclosures
Authors declare no conflicts of interest.
References
- 1. Files DK, Jausurawong T, Katrajian R, Danoff R. Multiple sclerosis. Prim Care 2015; 42:159–75. [DOI] [PubMed] [Google Scholar]
- 2. Pasquini MC, Griffith LM, Arnold DL et al Hematopoietic stem cell transplantation for multiple sclerosis: collaboration of the CIBMTR and EBMT to facilitate international clinical studies. Biol Blood Marrow Transplant 2010; 16:1076–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oliveira‐Rodriguez MA, Hamersschlak N, Aparecida‐de‐Moraes D, Pinto‐Simoes B, Rodrigues M, Feitosa‐Ribeiro AA, Voltarelli JC (in memoriam). Guidelines of the Brazilian Society of Bone Marrow Transplantation on hematopoietic stem cell multiple sclerosis. Rev Bras Hematol Hemoter 2013; 35:134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rogne S. Unethical for neurologists not to offer patients with multiple sclerosis chemotherapy with autologous stem cell support. Tidsskr Nor Laegeforen 2014; 134:1931–2. [DOI] [PubMed] [Google Scholar]
- 5. Stellmann JP, Sturner KH, Ufer F et al Stem cell transplantation for multiple sclerosis; Hamburg experiences and state of international research. Nervenarzt 2015; 86:989–96. [DOI] [PubMed] [Google Scholar]
- 6. Shevchenko JL, Kuztnetsov AN, Lonova TI et al Long‐term outcomes of autologous hematopoietic stem cell transplantation with reduced‐intensity conditioning in multiple sclerosis: Physician’s and patient’s perspectives. Ann Hematol 2015; 94:1149–55. [DOI] [PubMed] [Google Scholar]
- 7. Ruíz‐Argüelles GJ, León‐Peña AA, León‐González M et al A feasibility study of the full outpatient conduction of hematopoietic transplant in persons with multiple sclerosis employing autologous non‐cryopreserved peripheral blood stem cells. Acta Haematol 2017; 137:214–9. [DOI] [PubMed] [Google Scholar]
- 8. Gale RP, Gómez‐Cruz GB, Olivares‐Gazca JC et al Determine safety of outpatient chemotherapy and autotransplants using refrigerated, non‐frozen grafts in persons with multiple sclerosis. Clin Transplant 2019;e13567 10.1111/ctr.13567. [DOI] [PubMed] [Google Scholar]
- 9. Ruiz‐Argüelles GJ, Ruiz‐Argüelles A, Pérez‐Romano B, Marín‐López A, Larregina‐Díez A, Apreza‐Molina MG. Filgrastim‐mobilized peripheral‐blood stem cells can be stored at 4 degrees and used in autografts to rescue high‐dose chemotherapy. Am J Hematol 1995; 48:100–3. [DOI] [PubMed] [Google Scholar]
- 10. Ruiz‐Argüelles GJ, Ruiz‐Argüelles A, Pérez‐Romano B, Marín‐López A, Delgado‐Lamas JL. Non‐cryopreserved peripheral blood stem cells autotransplants for hematological malignancies can be performed entirely on an outpatient basis. Am J Hematol 1998; 58:161–4. [DOI] [PubMed] [Google Scholar]
- 11. Ruiz‐Argüelles GJ, Lobato‐Mendizábal E, Ruiz‐Argüelles A, Pérez‐Romano B, Arizpe‐Bravo D, Marín‐López A. Non‐cryopreserved unmanipulated hematopoietic peripheral blood stem cell autotrasplant program: Long term results. Arch Med Res 1999; 30:380–4. [DOI] [PubMed] [Google Scholar]
- 12. Gómez‐Almaguer D, Ruiz‐Argüelles GJ, Ruiz‐Argüelles A, González‐Llano O, Cantú OG, Hernández NE. Hematopoietic stem cell allografts using a non‐myeloablative conditioning regimen can be safely performed on an outpatient basis. Bone Marrow Transpl 2000; 25:131–3. [DOI] [PubMed] [Google Scholar]
- 13. Ruiz‐Argüelles GJ, Gómez‐Almaguer D, Ruiz‐Argüelles A, González‐Llano O, Cantú OG, Jaime‐Pérez JC. Results of an outpatient‐based stem cell allotransplant program using non‐myeloablative conditioning regimens. Am J Hematol 2001; 66:241–4. [DOI] [PubMed] [Google Scholar]
- 14. Ruiz‐Argüelles GJ, Ruiz‐Argüelles A, Gómez‐Almaguer D et al Features of the engraftment of allogeneic hematopoietic stem cells using reduced‐intensity conditioning regimens. Leukemia Lymph 2001; 42:145–50. [DOI] [PubMed] [Google Scholar]
- 15. Ruiz‐Argüelles GJ. Allogeneic stem cell transplantation using non‐myeloablative conditioning regimens: results of the Mexican approach. Int J Hematol 2002; 76(Suppl 1):376–9. [DOI] [PubMed] [Google Scholar]
- 16. Velázquez‐Sanchez‐de‐Cima S, Zamora‐Ortiz G, Ruiz‐Delgado GJ, Ruiz‐Argüelles GJ. Breaking another dogma: Successful hematopoietic stem cell transplantation in patients over 60 years of age: A single institution’s, 20‐year experience. Rev Hematol Méx 2013; 14:3–8. [Google Scholar]
- 17. López‐Otero A, Ruiz‐Delgado GJ, Ruiz‐Argüelles GJ. A simplified method for stem cell autografting in multiple myeloma: A single institution experience. Bone Marrow Transplant 2009; 44:715–9. [DOI] [PubMed] [Google Scholar]
- 18. Ruiz‐Argüelles GJ. Outpatient programs of myeloablative chemotherapy, autologous and allogeneic bone marrow transplantation. Haematologica 2000; 85:1233–4. [PubMed] [Google Scholar]
- 19. Ruiz‐Delgado GJ, Ruiz‐Argüelles GJ. A Mexican way to cope with stem cell transplantation. Hematology 2012; 17(Suppl 1):195–7. [DOI] [PubMed] [Google Scholar]
- 20. Gómez‐Cruz GB, Olivares‐Gazca M, Murrieta‐Alvarez I et al À‐propos of the 1000th stem cell transplant conducted at the Clínica Ruiz in Puebla. México. Rev Hematol Méx 2019; 20:150–83. [Google Scholar]
- 21. Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: Reliability, validity and guidelines. J Clin Oncol 1994; 2:187–193. [DOI] [PubMed] [Google Scholar]
- 22. Thomson A. ClinicSpeak: MS EDSS Calculator [internet]. [cited 2019 Jul 8]. Available at: https://edss.clinicspeak.com/en/#!/welcome (accessed 14 August 2019).
- 23. Kessinger A, Armitage JO, Landmark JD, Smith DM, Weisenburger DD. Autologous peripheral hematopoietic stem cell transplantation restores hemopoietic function following marrow ablative therapy. Blood 1988; 71:723–7. [PubMed] [Google Scholar]
- 24. Ruiz‐Argüelles A. Flow cytometry in the clinical laboratory. Principles, applications and problems. Ann Biol Clin 1992; 50:735–43. [PubMed] [Google Scholar]
- 25. Karduss‐Urueta A, Gale RP, Gutiérrez‐Aguirre CH et al Freezing the graft is not necessary for autotransplants for plasma cell myelomas and lymphoma. Bone Marrow Transplant 2018; 53:457–60. [DOI] [PubMed] [Google Scholar]
- 26. Ruiz‐Argüelles A, Gastélum‐Cano JM, Méndez‐Huerta MA, Rodríguez‐Gallegos AB, Ruiz‐Argüelles GJ. Glomerular filtration rate is impaired in patients with multiple sclerosis undergoing stem cell transplantation, and might be further deteriorated by cyclophosphamide. Lab Med 2019; 50:42–6. 10.1093/labmed/lmy028. [DOI] [PubMed] [Google Scholar]
- 27. DeZern AE, Petri M, Drachman DB et al High‐dose cyclophosphamide without stem cell rescue in 207 patients with aplastic anemia and other autoimmune diseases. Medicine (Balt) 2011; 90:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Collins CDE, Ivry B, Bowen JD et al A comparative analysis of patient‐reported expanded disability status scale tools. Mult Scler 2016; 22:1349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bowen J, Gibbons L, Gianas A, Kraft GH. Self‐administered expanded disability status scale with functional system scores correlates well with a physician‐administered test. Mult Scler 2001; 7:201–6. [DOI] [PubMed] [Google Scholar]
- 30. Muraro PA, Pasquini M, Atkins HL et al Long‐term Outcomes Study Group. Long‐term outcomes after autologous hematopoietic stem cell transplantation for multiple sclerosis. JAMA Neurol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sormani MP, Muraro PA, Saccardi R, Mancardi G. NEDA status in highly active MS can be more easily obtained with autologous hematopoietic stem cell transplantation than other drugs. Mult Scler 2016: 1–4. [DOI] [PubMed] [Google Scholar]
- 32. Ruiz‐Argüelles GJ, Gómez‐Almaguer D. Hematopoietic stem cell transplants for persons with multiple sclerosis: is this the best therapeutic option? Medicina Univ 2017; 19:208–9. [Google Scholar]
- 33. Hauser SL, Bar‐Or A, Comi G et al Ocrelizumab versus interferon beta‐1a in relapsing multiple sclerosis. N Engl J Med 2017; 376:221–34. [DOI] [PubMed] [Google Scholar]
- 34. Burt RK, Balabanov R, Burman J et al Effect of nonmyeloablative hematopoietic stem cell transplantation vs continued disease‐modifying therapy on disease progression in patients with relapsing‐remitting multiple sclerosis: a randomized clinical trial. JAMA 2019; 321:165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cohen JA, Baldassari LE, Atkins HL et al Autologous hematopoietic cell transplantation for treatment‐refractory relapsing multiple sclerosis: Position statement from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2019; 25:845–54. [DOI] [PubMed] [Google Scholar]


