Figure 3.
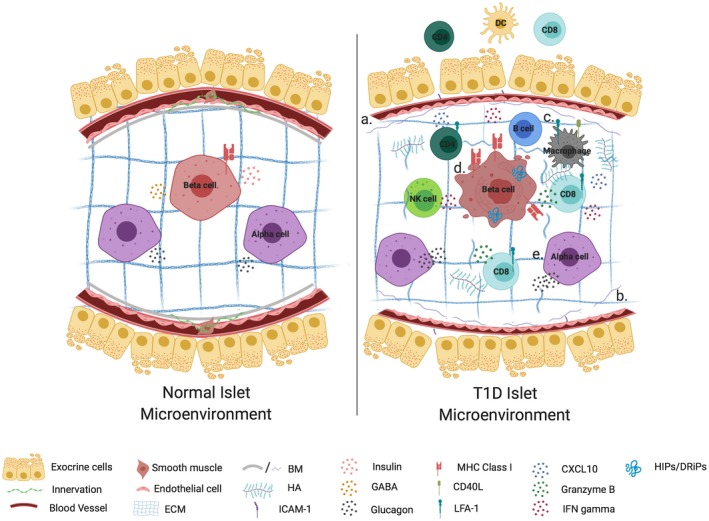
Islet–immune interactions and their impact on β‐cell function in health and T1D. An islet in a normal microenvironment is surrounded by intact extracellular matrix and exocrine tissue, while provided sufficient vascularization and innervation to promote glucose homeostasis and immunoregulation through insulin and glucagon secretion and release of GABA (left). In T1D (a) reduced vessel diameter and innervation impair islet‐cell function, while morphological dysfunction in the form of (b) loss of basement membrane (BM) and extracellular matrix (ECM) integrity as well as hyaluronan (HA) accumulation permit (c) the infiltration and activation of lymphocytes in both the exocrine and intra‐islet tissue. These immune cells make use of chemokine gradients and promote a diabetogenic milieu through the release of pro‐inflammatory mediators. Cumulatively these defects contribute toward β‐cell dysfunction in the form of (d) MHC class I hyperexpression, loss of insulin and GABA expression. They also may serve to magnify endogenous β‐cell stress presented as protein processing defects, which in turn function as neoantigens to exacerbate immune activation, all of which culminate in β‐cell death. In addition to β‐cell defects, α‐cell dysfunction (e) contributes to impaired glucose homeostasis in T1D through hyperglucagonemia or failed counterregulatory responses. Figure created with Biorender.
