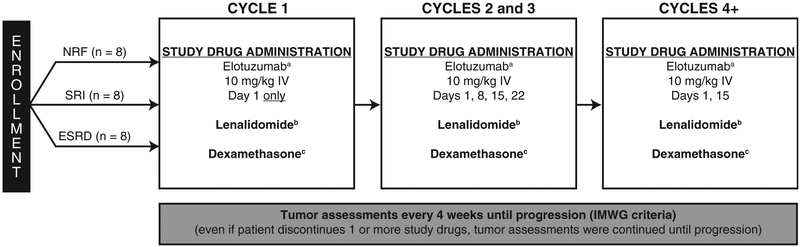
Figure 1.
Study Design Showing the Number of Patients Planned for Enrollment. aPremedication With an H1 Blocker (Diphenhydramine, 25–50 mg, or Equivalent), an H2 Blocker (Ranitidine, 50 mg, Adjusted for Renal Failure, or Equivalent), and Acetaminophen (650–1000 mg) Was Required 30–90 Minutes Before Elotuzumab Administration. bIn All Patients, Lenalidomide Was Given Daily for 21 Days of a 28-day Cycle: Normal Renal Function (NRF), 25 mg Orally (p.o.) Once Daily; Severe Renal Impairment (SRI), 15 mg p.o. Every 48 Hours; End-Stage Renal Disease (ESRD), 5 mg p.o. Once Daily. cWeeks Without Elotuzumab: 40 mg p.o.; Weeks With Elotuzumab 8 mg Intravenously (I.V.) Plus 28 mg p.o.
Abbreviation: IMWG = International Myeloma Working Group.