Figure 2.
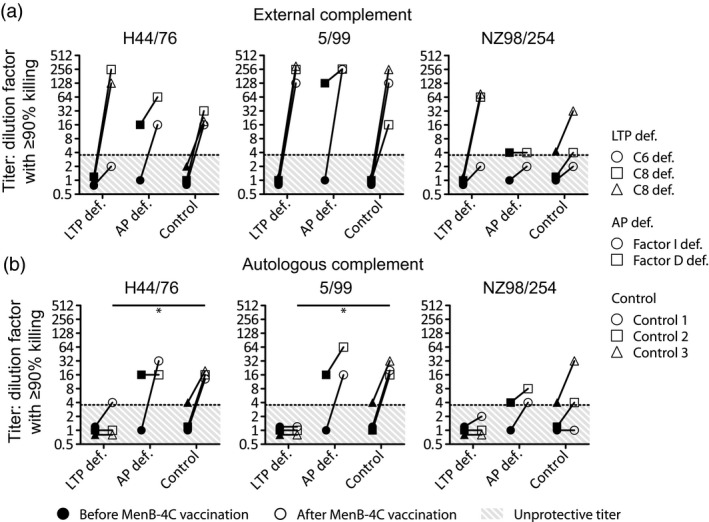
Exogenous human complement source serum bactericidal activity assay with sera from complement‐deficient patients or controls. Sera were collected from three patients with a late terminal complement pathway (LTP def.); C6 deficiency (○) or C8 deficiency (Δ and □) or two patients with alternative pathway deficiency (AP def.); factor I deficiency (○) or factor D deficiency (□) before (filled symbols) and after (open symbols) vaccination with multi‐component meningococcal serogroup B (MenB)‐4C. Classical serum bactericidal activity assay with exogenous human serum was determined (a). Serum bactericidal activity assay with autologous human serum was determined (b). Titers were based on the initial serum dilution that showed 90% or more killing. Results with a P‐value of < 0·05 were considered significant and are marked with an asterisk. Pre‐vaccination titers were compared between groups. The same was performed for post‐vaccination titers.
