Abstract
Objectives
Linezolid is an important therapeutic option for the treatment of infections caused by VRE. Linezolid is a synthetic antimicrobial and resistance to this antimicrobial agent remains relatively rare. As a result, data on the comparative genomics of linezolid resistance determinants in Enterococcus faecium are relatively sparse.
Methods
To address this knowledge gap in E. faecium, we deployed phenotypic antibiotic susceptibility testing and Illumina WGS on hospital surface (environmental) and clinical isolates from the USA and Pakistan.
Results
We found complete concordance between isolate source country and mechanism of linezolid resistance, with all the US isolates possessing a 23S rRNA gene mutation and the Pakistan isolates harbouring two to three acquired antibiotic resistance genes. These resistance genes include the recently elucidated efflux-pump genes optrA and poxtA and a novel cfr-like variant. Although there was no difference in the linezolid MIC between the US and Pakistan isolates, there was a significant difference in the geometric mean of the MIC between the Pakistan isolates that had two versus three of the acquired antibiotic resistance genes. In five of the Pakistan E. faecium that possessed all three of the resistance genes, we found no difference in the local genetic context of poxtA and the cfr-like gene, but we identified different genetic contexts surrounding optrA.
Conclusions
These results demonstrate that E. faecium from different geographical regions employ alternative strategies to counter selective pressure of increasing clinical linezolid use.
Introduction
Enterococcus faecium is a common gut commensal organism and an increasingly important cause of nosocomial infection.1 One feature implicated in the success of E. faecium as a pathogen is its repertoire of acquired antibiotic resistance genes (ARGs) that enable evasion of antimicrobial therapy.1 As an example, treatment of E. faecium infections with vancomycin has facilitated proliferation of the vanA gene cassette throughout E. faecium.2 Due to the increase in vancomycin-resistant Gram-positive pathogens, newer therapeutics, notably the oxazolidinones linezolid and tedizolid, have become important therapeutic agents for treating infections caused by this organism.3
Accordingly, sporadic resistance to linezolid has been identified in cohorts of E. faecium and other Gram-positive bacteria.4–6 These include vertically transmitted mutations in the linezolid target, the 23S rRNA gene sequence, and alterations in the ribosomal proteins L3, L4 and L22.7–9 Acquired plasmid-borne ARGs, including the 23S rRNA methyltransferases cfr and cfr(B), have been previously identified in E. faecium.10–12 Newly identified efflux-pump genes, optrA and poxtA, have also been described in E. faecium.13,14
Despite the identification of vertically and horizontally transferable linezolid resistance determinants, a comprehensive genomic survey of linezolid-resistant E. faecium isolates has not been performed. Additionally, there is a gap in knowledge on the relationship of established linezolid resistance determinants and their encoded phenotypic susceptibility to the newest oxazolidinone, tedizolid. To address this, we performed WGS and comparative analysis on 41 newly sequenced isolates from the USA and 8 newly sequenced isolates from Pakistan. To increase the number of isolates for analysis, we supplemented these data with 52 publicly available genomes of E. faecium isolated from the same locations in the USA and Pakistan. Our results indicate that the mechanism of linezolid resistance is more strongly associated with geography rather than E. faecium clade/phylogeny in this cohort, with resistant isolates from the USA harbouring the G2576T SNP in 23S rRNA loci and resistant isolates from Pakistan encoding combinations of poxtA, optrA and cfr-like ARGs.
Materials and methods
Linezolid-non-susceptible E. faecium cohort
To understand the genotypic mechanism for linezolid resistance in two different geographies, we analysed a collection of banked linezolid-intermediate and linezolid-resistant E. faecium isolates recovered from cultures of environmental or clinical specimens between 2012 and 2018. Inclusion criteria include phenotypic resistance or intermediate resistance to linezolid using the Etest gradient diffusion assay (bioMérieux, Durham, NC, USA). We accessed 44 banked linezolid-non-susceptible environmental E. faecium and 3 linezolid-susceptible isolates from 2015 to 2016 that were sequenced in a previous analysis (BioProject PRJNA497126) from longitudinal surveillance of hospital surfaces in Pakistan. We newly sequenced four linezolid-non-susceptible and four linezolid-susceptible isolates collected from a previous analysis of clinical isolates obtained in 2012–13 from two hospitals in Pakistan.15 We additionally accessed 30 clinical isolates of linezolid-non-susceptible E. faecium banked from the clinical microbiology laboratory of Barnes-Jewish Hospital (St Louis, MO, USA) from 2015 to 2018. Finally, we accessed eight environmental linezolid-non-susceptible and three linezolid-susceptible E. faecium isolates obtained from environmental surfaces in the Barnes-Jewish Hospital during 2017–18. E. faecium Aus0004 (clade A1 reference), E. faecium E2134 (clade A2 reference) and E. faecium E1007 (clade B reference) were obtained from a previous genomic analysis of Enterococcus evolution.16 The linezolid-resistant isolate due to a 23S rRNA G2576T mutation, E. faecium VRE1558, and the linezolid-resistant isolate due to a 23S rRNA G2505A mutation, E. faecium E1644, were also included in the phylogenetic analysis.17,18
Illumina WGS and genomic analysis
Stock cultures of the E. faecium isolates sequenced in this investigation were recovered from freezer vials and streaked out onto blood agar (Hardy Diagnostics). Approximately 10 colonies were suspended into 1 mL of nuclease-free water. Genomic DNA was extracted using the QIAamp BiOstic Bacteremia DNA Kit (QIAGEN, Germantown, MD, USA). Genomic DNA was sequenced with Illumina WGS, producing short-read sequences. Illumina adapter sequences were removed using Trimmomatic (version 0.38) and sequence contamination was removed with DeconSeq (version 0.4.3).19,20 The processed reads were assembled into contigs using SPAdes (version 3.13.0).21 Isolates sequenced in this paper, as well as previously sequenced isolates (including outgroups E1007, Aus0004 and E2134, used for clade identification, and VRE1558 and E1644, positive for 23S rRNA mutations G2576T and G2505A, respectively), were annotated with Prokka (version 1.12).22 MLST STs were also determined using BLAST similarity (https://github.com/tseemann/mlst). Core-genome analysis was performed with Roary (version 3.12.0) on the .gff files from Prokka. The core-genome alignment with PRANK was converted to an approximate maximum-likelihood tree in FastTree (version 2.1.9). After determination that all of the isolates were from clades A1 or A2 we removed the clade B genome from analysis and performed parSNP (version 1.2) on the FASTA files of the isolates.23 The Newick file for both trees were viewed in iTOL.24 For detailed information on software parameters and commands used in this investigation, please see Appendix S1 (available as Supplementary data at JAC Online).
Antibiotic susceptibility testing
Pure cultures of isolates had phenotypic antibiotic resistance determined using Kirby–Bauer disc diffusion assays and gradient diffusion (e.g. Etest) assays. Both assays were performed according to the manufacturers’ instructions. The results were interpreted using the CLSI M100 criteria for Enterococcus species.25 Linezolid (BD, Franklin Lakes, NJ, USA) and vancomycin (Hardy Diagnostics, Santa Maria, CA, USA) were tested using Kirby–Bauer discs. Strains were classified as linezolid susceptible at or above 23 mm, intermediate at 21–22 mm and resistant at or below 20 mm; similarly, strains were classified as vancomycin susceptible at or above 17 mm, intermediate at 15–16 mm and resistant at or below 14 mm. We additionally tested linezolid (bioMérieux), daptomycin (bioMérieux), dalbavancin (Liofilchem, Waltham, MA, USA) and tedizolid (Liofilchem) using quantitative gradient diffusion assays and interpreted the MIC value in accordance with 2019 CLSI standards: strains were classified as linezolid susceptible at or below 2 mg/L, intermediate at 4 mg/L and resistant at or above 8 mg/L; strains were classified as daptomycin susceptible at or below 1 mg/L, susceptible dose-dependent at 2–4 mg/L and resistant at or above 8 mg/L; and strains were classified as dalbavancin susceptible at or below 0.25 mg/L.25 As there is currently an absence of E. faecium breakpoints for tedizolid, we used the Enterococcus faecalis breakpoint criteria for our cohort; strains were classified as tedizolid susceptible at or below 0.5 mg/L and non-susceptible above 0.5 mg/L. All interpretations of Etest MIC values were performed with clinical accuracy and read appropriately. Reported Etest MIC values were rounded up to the nearest doubling dilution.
In silico oxazolidinone resistance determinant identification
ResFinder annotation of known resistance genes was used to identify isolates that harboured optrA, poxtA and vanX.26 We used Roary to assemble the pangenome of the isolates and found that a cfr-like gene had been annotated in the genes_presence_absence output of the program.27 The gene sequence was compared with cfr and variant cfr(B) sequences using BLAST.11,28
Following published suggestions for determining linezolid resistance mutations, the reads of processed isolates were aligned using Bowtie2 to a reference 23S rRNA sequence of Aus0004.29 The 23S rRNA sequence of Aus0004 (NCBI Reference Sequence: NR_103056.1) did not harbour any of the mutations associated with linezolid resistance. SNPs that did not match the Aus0004 reference sequence were identified using a custom Python 3 script. From this alignment, the site of the SNP that correlated with the G2576T mutation (using Escherichia coli numbering) responsible for linezolid resistance was identified. Isolates found to be positive for the mutation by this method had the SNP in at least 50% of reads. To identify all isolates that had the G2576T mutation at any frequency, a second script was run to extract isolates with an SNP at the respective site. All isolates having the mutation at a frequency of at least 17% of reads, which is regarded as the minimum frequency for phenotypic linezolid resistance, were considered to be resistant by ribosomal mutation.29 Other published mutations responsible for linezolid resistance were sought out, but not identified in any of the isolates; these included the G2505 23S rRNA gene mutation and mutations in the L3, L4 and L22 proteins.18,30,31
Data availability
All genomes sequenced in this study have been uploaded to the NCBI WGS database associated with BioProject PRJNA517335.
Results
Acquired linezolid resistance genes (optrA, poxtA and cfr-like) were found exclusively in the E. faecium isolates recovered from Pakistan, regardless of clade
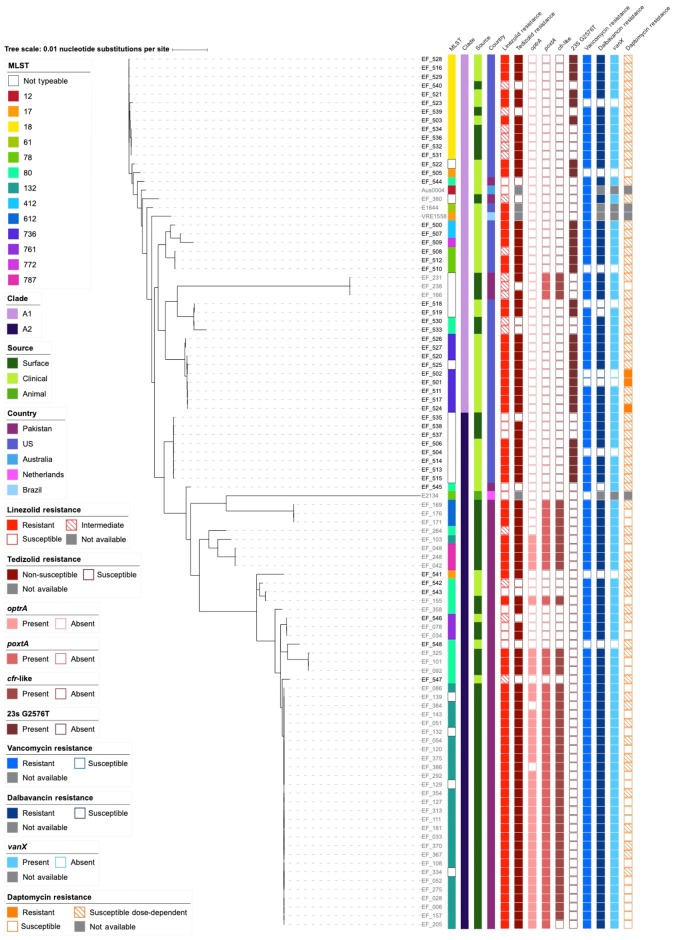
We accessed banked environmental and clinical isolates of linezolid-non-susceptible E. faecium isolates from the USA and Pakistan as well as several known linezolid-susceptible isolates from both locations to perform genomic analysis of linezolid resistance determinants. We used Illumina WGS to construct draft genomes for 49 isolates (Table S1) and obtained 52 publicly available E. faecium genomes isolated from the same locations in the USA and Pakistan (Table S2). We used Kirby–Bauer disc diffusion and gradient diffusion methods in conjunction with CLSI interpretive guidelines to assign phenotypic resistance criteria to linezolid (resistant, intermediate or susceptible) and tedizolid (using E. faecalis breakpoints for non-susceptible or susceptible). Initially, we constructed a core-genome phylogenetic tree on the 1691 core genes between all genomes. Phylogenetic comparison of the cohort with reference isolates from E. faecium clades A1, A2 and B determined that all isolates in the cohort belong to clades A1 and A2, characteristic of human pathogens (Figure S1).16 To gain further resolution on the relatedness of the E. faecium isolates, we excluded the clade B isolate E1007 and constructed a recombination-free phylogenetic tree using parSNP (Figure 1). The phylogeny of the isolates was generally geographically stratified, as 80.5% (33/41) of E. faecium from the USA were in clade A1 and 90.9% (50/55) of E. faecium from Pakistan were in clade A2. The isolate cohort represented 11 identifiable MLST STs. Of the US isolates, 70.7% (29/41) were resistant to linezolid and, of these, 100% (29/29) were positive for the G2576T 23S rRNA SNP using Bowtie2 alignment of Illumina reads to the Aus0004 reference sequence (Figure 1).29 A comparable proportion of the E. faecium isolates from Pakistan, 72.7% (40/55), were also resistant to linezolid; however, in contrast, 97.5% (39/40) of these isolates were positive for an acquired linezolid resistance gene identified by ResFinder or Prokka, but negative for the G2576T SNP. The canonical 23S rRNA methyltransferase gene cfr was not identified in our isolates; however, a variant of the cfr family was annotated by Prokka in 76.4% (42/55) of E. faecium isolates from Pakistan (Figure 1). BLASTP query and comparison with previously characterized sequences of the cfr gene, the cfr(B) variant and the ancestral rlmN gene determined that the cfr-like gene shared 64% identify over 95% of query length with the original cfr gene and 65% identity over 97% of the length of cfr(B) (Figure S2). An identity of 74.9% over 99.7% was previously used to classify cfr(B) as unique from cfr, therefore the gene we have described fits within the category of other emerging cfr-like family members.32,33 Of the isolates from Pakistan, 78.2% (43/55) and 61.8% (34/55) contained the linezolid ABC transporters poxtA and optrA, respectively. Of the isolates with gene-based resistance, 76.7% (33/43) harboured all three of the resistance genes identified in the cohort; 20.9% (9/43) of the isolates harboured only poxtA and the cfr-like gene and 2.33% (1/43) harboured only optrA and poxtA. Of the isolates from both Pakistan and the USA, 90.6% (87/96) and 88.5% (85/96) of the isolates were resistant to vancomycin and dalbavancin, respectively. Only 3.13% (3/96) of the isolates were resistant to daptomycin, another therapeutic agent commonly used to treat VRE in the USA; however, an additional 68.8% (66/96) had MIC values in the susceptible dose-dependent classification range. These results indicate that while clade A1 and clade A2 E. faecium isolates can be found in both the USA and Pakistan, there is a differential burden in the mechanism of linezolid resistance between the surveyed isolates from these locations.
Figure 1.
Recombination-free phylogenetic tree including MLST, country, source, resistance, resistance gene and mutation data. Linezolid resistance in US isolates was attributed solely to the G2576T mutation of the 23S rRNA gene sequence. In contrast, linezolid resistance in Pakistan isolates resulted from different combinations of the acquired resistance genes optrA, poxtA and a cfr-like gene. Vancomycin resistance was observed in 90.6% (87/96) of the isolates and dalbavancin resistance was observed in 88.5% (85/96). Daptomycin resistance was observed in 3.13% (3/96) of the isolates with an additional 68.8% (66/96) classified as susceptible dose-dependent.
Linezolid resistance differs by genes present, not by mechanism
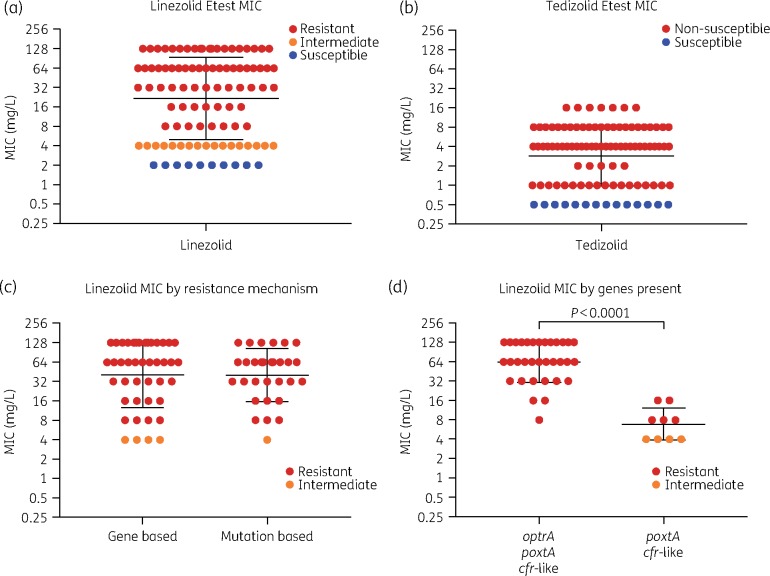
The geometric mean linezolid MIC (21.83 mg/L) was greater than the geometric mean tedizolid MIC (2.87 mg/L) (Figure 2a and b). There was minimal difference between the geometric mean linezolid MIC for isolates with gene-based linezolid resistance (40.75 mg/L) and isolates with mutation-based linezolid resistance (40.32 mg/L) (Figure 2c). However, the geometric mean linezolid MIC for isolates with all three observed resistance genes (64 mg/L) was significantly greater (P<0.0001) than the geometric mean linezolid MIC for isolates that harboured only poxtA and the cfr-like gene (6.86 mg/L) (Figure 2d). Our results demonstrate that while tedizolid resistance and linezolid resistance may be related, there are several instances in our cohort where they are independent of one another (Figure S3). Of the 96 isolates, 22 (22.9%) were neither susceptible to both antibiotics nor resistant to linezolid and non-susceptible to tedizolid (Figure S3). Of these, 40.9% (9/22) of isolates had intermediate linezolid resistance, but were susceptible to tedizolid, 36.4% (8/22) of isolates were linezolid intermediate and non-susceptible to tedizolid and 22.7% (5/22) of isolates were susceptible to linezolid, but non-susceptible to tedizolid (Figure S3). The previously identified 23S rRNA G2505A linezolid resistance mutation was not identified within the isolates from our cohort.18,29 However, heterogeneity at site 1232 in the aligned 23S rRNA gene of E. faecium Aus0004 was observed in all isolates from our cohort (with >17% frequency in 76 isolates). This site has not previously been associated with linezolid resistance and the mutation was observed in both linezolid-resistant and -susceptible isolates, therefore it likely does not contribute to phenotypic linezolid resistance. Within the population of E. faecium that contained the G2576T mutation at >17%, there was not a correlation between frequency of the G2576T SNP and phenotypic linezolid resistance (Figure S4).
Figure 2.
Linezolid and tedizolid MICs and comparisons by basis of resistance mechanism. The geometric mean MIC of linezolid (a) is higher than the geometric mean MIC of tedizolid (b) at 21.83 and 2.87 mg/L, respectively. There was no difference in linezolid resistance between isolates with gene- or mutation-based resistance mechanisms (c). However, isolates that harboured poxtA and cfr-like genes had significantly lower levels of linezolid resistance than those that harboured all three linezolid resistance genes (d); statistical analysis was done using the unpaired t-test in Prism v8. Please note, y-axis values for all graphs are log2 scaled for visual acuity.
Different genetic platforms of optrA in linezolid-resistant E. faecium from Pakistan
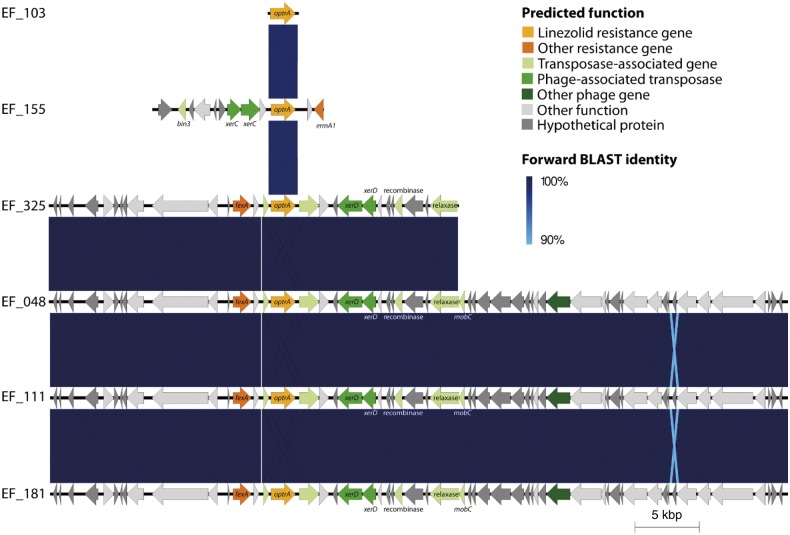
We used EasyFig to analyse the genetic context of optrA, poxtA and the cfr-like gene in five isolates that harboured all three genes (Figure 3). The visualized genetic context of optrA was identical in Pakistan isolates EF_48, EF_111 and EF_181, as well as partially in EF_325. These segments harboured a fexA phenicol resistance gene adjacent to optrA. The context of optrA in EF_155 differed from the others and contained the erm(A1) methyltransferase gene. The optrA contigs also contained several transposase-associated and phage-associated transposase genes, which could enable horizontal transfer of the optrA gene. The contig from EF_103 contained only the optrA gene. In all cases poxtA was assembled on a short contig with no other flanking genes and the genetic context around the cfr-like gene was identical in the isolates we observed (Figure S5).
Figure 3.
Genetic context of optrA in isolates that harbour optrA, cfr-like and poxtA genes. In isolates EF_325, EF_048, EF_111 and EF_181, optrA is downstream of the resistance gene fexA and in isolate EF_155 it is upstream of an erm(A1) resistance gene. These contexts are similar to those that optrA was in when it was first identified. However, the mobile elements surrounding optrA in our isolates differ from those previously identified. optrA’s location near mobile elements may allow it to be transferable.
Discussion
The molecular epidemiology of linezolid resistance in VRE is largely uncharacterized, but linezolid resistance is rapidly increasing.5 Consistent with earlier reports on the distribution of isolates in E. faecium clades, all of our isolates were in the A1 or A2 group.16 Nearly 72% (69/96) of the isolates in this study were linezolid resistant, with an additional 18% (17/96) having intermediate linezolid resistance. Additionally, 85% (82/96) of the isolates were non-susceptible to tedizolid, with much lower MIC values than observed for linezolid, as has been previously observed in linezolid-resistant E. faecium from Germany.34 In our cohort, linezolid resistance can be attributed to a combination of resistance genes or the G2576T mutation in the 23S rRNA gene. While the resistance mechanism differs between geographical locations, with resistance in the strains recovered from Pakistan containing gene-mediated resistance determinants and US isolates harbouring 23S rRNA gene mutation(s), both groups displayed similar phenotypic MIC distributions. Possibly due to differences between short-read Illumina and longer-read Sanger sequencing, we did not observe a correlation between the linezolid MIC and the proportion of the G2576T mutation 23S rRNA allele, as has been identified previously.35 Limiting linezolid use may partly curtail the spread of resistance, as the G2576T resistance mutation can arise in pathogens due to prolonged drug exposure and the cfr, optrA and poxtA resistance genes identified have historically been capable of horizontal transfer through situation on mobile genetic elements.14,36–38 Tedizolid holds promise for treatment of MDR infections.39 However, we found that 100% (69/69) of linezolid-resistant isolates were also non-susceptible to tedizolid and 47% (8/17) of linezolid-intermediate isolates were tedizolid non-susceptible. Unexpectedly, five isolates were linezolid susceptible but tedizolid non-susceptible, although the MIC distributions for these isolates were near the resistance breakpoint for both antimicrobials. The MIC breakpoints published by the CLSI for non-susceptibility to tedizolid are lower than for linezolid based on pharmacokinetic and pharmacodynamic properties.40 Future investigations to examine tedizolid-specific resistance determinants and suitable breakpoints specifically for E. faecium are warranted.39
To the best of our knowledge, the cfr 23S rRNA methyltransferase family and the optrA and poxtA efflux pump genes are the only known acquired ARGs against linezolid.10,13,14 These genes can also confer resistance to other antibiotics, including chloramphenicol and clindamycin, complicating treatment options. cfr, cfr(B), cfr(C) and unnamed cfr-like genes have previously been identified in linezolid-resistant strains of Staphylococcus aureus, Clostridioides (Clostridium) difficile, Enterococcus spp., E. faecalis and E. faecium.10,11,41–44 Interestingly, these genes do not appear to be restricted to pathogens, but can be found in a diverse number of Gram-positive species, indicating that multiple opportunities for horizontal gene transfer may arise.33 Previously, cfr and its variants have been identified in isolates from countries including the USA, Germany, Spain, Italy, China, France, Denmark and the UK, but, to the best of our knowledge, this is the first report from Pakistan. In all isolates in which we observed the cfr-like gene, we also identified poxtA or both poxtA and optrA. Among isolates that only harboured the cfr-like gene and poxtA, the geometric mean MIC (6.86 mg/L) was ∼ 10 times lower than that for those that harboured all three identified resistance genes (64 mg/L), with one of the two-gene isolates achieving only intermediate resistance. The genes optrA and cfr have previously been reported co-localized on plasmids in hospital-borne vancomycin-resistant E. faecium.45 Upon its discovery, there was doubt as to whether cfr(B) granted the same resistance phenotype in Enterococcus as it does in Staphylococcus or if the cfr-like gene from C. difficile also confers antibiotic resistance.11,46 Additionally, a recent study using a mouse peritonitis model found that tedizolid underperformed compared with linezolid and daptomycin in bacterial clearance of cfr(B)-positive E. faecium.47 Treatment of cfr(B)-positive E. faecium infection with linezolid garnered 86% survival in a mouse peritonitis model, despite presenting MICs that would suggest linezolid resistance.47 Our data, coupled with these observations, suggest that the relative contribution of the cfr-like gene to phenotypic resistance may be less significant than that of other resistance genes and could be attributed to significant genotypic divergence from the canonical cfr gene. These phenotypic discrepancies may be exacerbated by synergistic effects occurring between the optrA and poxtA transporters and the cfr-like methyltransferase that are not occurring when poxtA and the cfr-like gene contribute to resistance in the absence of optrA. Therefore, while it is possible the cfr-like gene, poxtA and optrA contribute equally to linezolid resistance, further investigation is necessary to determine their individual impacts on the observed resistance phenotypes.
Notably, optrA resided in different contexts within our isolates. Comparing the ARG genetic contexts of isolates randomly selected from different branches of the phylogenetic tree, we found several isolates with contexts similar to those in which optrA was originally identified—having either the fexA phenicol exporter gene upstream of optrA or an erm(A1) ARG downstream of optrA (Figure 3).37 However, the mobile elements identified in our isolates (several of which are phage-associated) differed from those previously observed near optrA. Although the limitations of short-read sequencing prevented us from obtaining longer genetic contexts of the poxtA and cfr-like genes (Figure S5), poxtA, optrA and cfr variants have previously been observed near mobilizing elements, with the cfr variants and optrA residing on plasmids.10,11,14,37
This study aimed to characterize the molecular epidemiology and investigate the differential burden of linezolid resistance mechanisms in E. faecium from two geographically distinct locations. We found that all US isolates have the 23S rRNA G2576T mutation, while isolates from Pakistan harbour combinations of a cfr-like gene, optrA and poxtA. While geometric mean MIC values for these groups did not differ greatly (40.75 mg/L for gene-based resistance and 40.32 mg/L for mutation-based resistance), there was a difference between isolates that harboured poxtA and optrA compared with those isolates that had all three putative ARGs. Daptomycin is the antimicrobial agent evaluated in this study with the highest rate of susceptibility based on in vitro testing; 3.13% (3/96) of isolates in this study are phenotypically resistant; however, 68.8% (66/96) of isolates are susceptible dose-dependent to daptomycin. Of note, daptomycin therapy is not a viable option for pulmonary infections, but Enterococcus spp. are very uncommon causes of pneumonia.48,49 Additionally, in the case of isolate EF_524, therapeutic options would be extremely limited as the isolate is resistant to linezolid, tedizolid, vancomycin, dalbavancin, daptomycin and ampicillin, the primary antibiotics available for Enterococcus infection treatment. In five isolates that harboured all three ARGs, optrA was observed in different genetic contexts, while the cfr-like gene and poxtA were observed in similar contexts or were assembled on contigs that were too short to identify flanking genes. The major limitation of this study is that by using Illumina sequencing we are unable to resolve plasmid versus chromosomal segments. The use of long-read sequencing may further provide context for the genetic environment surrounding cfr, poxtA and optrA in the isolates from Pakistan. Nevertheless, our results indicate that E. faecium isolates can use distinct genetic strategies to achieve comparable in vitro linezolid resistance. Continued investigation of linezolid resistance in E. faecium and antibiotic stewardship of linezolid are advised to prevent the spread of resistance to this last-resort antibiotic.
Supplementary Material
Acknowledgements
We thank members of the Dantas lab for insightful discussions of the results and conclusions. We thank the following staff at The Edison Family Center for Genome Sciences & Systems Biology for technical support: Eric Martin, Brian Koebbe, Jessica Hoisington-Lopez and MariaLynn Jaeger.
Funding
This work was supported by a United States Agency for International Development award (award number 3220–29047) to S. A., C.-A. D. B. and G. D. This work was supported in part by awards to G. D. through the National Institute of Allergy and Infectious Diseases and the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (NIH) under award numbers R01AI123394 and R01HD092414, respectively. R. F. P. received support from the Monsanto Excellence Fund Graduate Fellowship. A. W. D. received support from the Institutional Program Unifying Population and Laboratory-Based Sciences Burroughs Wellcome Fund grant to Washington University.
Transparency declarations
None to declare.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Supplementary data
Appendix S1, Tables S1 and S2 and Figures S1 to S5 are available as Supplementary data at JAC Online.
References
- 1. Miller WR, Munita JM, Arias CA.. Mechanisms of antibiotic resistance in enterococci. Expert Rev Anti Infect Ther 2014; 12: 1221–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Freitas AR, Tedim AP, Francia MV. et al. Multilevel population genetic analysis of vanA and vanB Enterococcus faecium causing nosocomial outbreaks in 27 countries (1986–2012). J Antimicrob Chemother 2016; 71: 3351–66. [DOI] [PubMed] [Google Scholar]
- 3. Bozdogan B, Appelbaum PC.. Oxazolidinones: activity, mode of action, and mechanism of resistance. Int J Antimicrob Agents 2004; 23: 113–9. [DOI] [PubMed] [Google Scholar]
- 4. Auckland C, Teare L, Cooke F. et al. Linezolid-resistant enterococci: report of the first isolates in the United Kingdom. J Antimicrob Chemother 2002; 50: 743–6. [DOI] [PubMed] [Google Scholar]
- 5. Bi R, Qin T, Fan W. et al. The emerging problem of linezolid-resistant enterococci. J Glob Antimicrob Resist 2018; 13: 11–9. [DOI] [PubMed] [Google Scholar]
- 6. Kumar S, Bandyoapdhyay M, Chatterjee M. et al. The first linezolid-resistant Enterococcus faecium in India: high level resistance in a patient with no previous antibiotic exposure. Avicenna J Med 2014; 4: 13–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stefani S, Bongiorno D, Mongelli G. et al. Linezolid resistance in staphylococci. Pharmaceuticals (Basel) 2010; 3: 1988–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ikonomidis A, Grapsa A, Pavlioglou C. et al. Accumulation of multiple mutations in linezolid-resistant Staphylococcus epidermidis causing bloodstream infections; in silico analysis of L3 amino acid substitutions that might confer high-level linezolid resistance. J Chemother 2016; 28: 465–8. [DOI] [PubMed] [Google Scholar]
- 9. Dong W, Chochua S, McGee L. et al. Mutations within the rplD gene of linezolid-nonsusceptible Streptococcus pneumoniae strains isolated in the United States. Antimicrob Agents Chemother 2014; 58: 2459–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morales G, Picazo JJ, Baos E. et al. Resistance to linezolid is mediated by the cfr gene in the first report of an outbreak of linezolid-resistant Staphylococcus aureus. Clin Infect Dis 2010; 50: 821–5. [DOI] [PubMed] [Google Scholar]
- 11. Deshpande LM, Ashcraft DS, Kahn HP. et al. Detection of a new cfr-like gene, cfr(B), in Enterococcus faecium isolates recovered from human specimens in the United States as part of the SENTRY Antimicrobial Surveillance Program. Antimicrob Agents Chemother 2015; 59: 6256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doern CD, Park JY, Gallegos M. et al. Investigation of linezolid resistance in staphylococci and enterococci. J Clin Microbiol 2016; 54: 1289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Y, Lv Y, Cai J. et al. A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J Antimicrob Chemother 2015; 70: 2182–90. [DOI] [PubMed] [Google Scholar]
- 14. Antonelli A, D’Andrea MM, Brenciani A. et al. Characterization of poxtA, a novel phenicol-oxazolidinone-tetracycline resistance gene from an MRSA of clinical origin. J Antimicrob Chemother 2018; 73: 1763–9. [DOI] [PubMed] [Google Scholar]
- 15. Pesesky MW, Hussain T, Wallace M. et al. KPC and NDM-1 genes in related Enterobacteriaceae strains and plasmids from Pakistan and the United States. Emerg Infect Dis 2015; 21: 1034–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lebreton F, Manson AL, Saavedra JT. et al. Tracing the enterococci from Paleozoic origins to the hospital. Cell 2017; 169: 849–61.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. do Prado GVB, Marchi AP, Moreno LZ. et al. Virulence and resistance pattern of a novel sequence type of linezolid-resistant Enterococcus faecium identified by whole-genome sequencing. J Glob Antimicrob Resist 2016; 6: 27–31. [DOI] [PubMed] [Google Scholar]
- 18. Prystowsky J, Siddiqui F, Chosay J. et al. Resistance to linezolid: characterization of mutations in rRNA and comparison of their occurrences in vancomycin-resistant enterococci. Antimicrob Agents Chemother 2001; 45: 2154–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bolger AM, Lohse M, Usadel B.. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 2014; 30: 2114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schmieder R, Edwards R.. Fast identification and removal of sequence contamination from genomic and metagenomic datasets. PLoS One 2011; 6: e17288.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bankevich A, Nurk S, Antipov D. et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 2012; 19: 455–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics 2014; 30: 2068–9.24642063 [Google Scholar]
- 23. Treangen TJ, Ondov BD, Koren S. et al. The Harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol 2014; 15: 524.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Letunic I, Bork P.. Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics 2007; 23: 127–8. [DOI] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing—Twenty-Ninth Edition: M100. CLSI, Wayne, PA, USA, 2019. [Google Scholar]
- 26. Zankari E, Hasman H, Cosentino S. et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 2012; 67: 2640–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Page AJ, Cummins CA, Hunt M. et al. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015; 31: 3691–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Altschul SF, Gish W, Miller W. et al. Basic local alignment search tool. J Mol Biol 1990; 215: 403–10. [DOI] [PubMed] [Google Scholar]
- 29. Beukers AG, Hasman H, Hegstad K. et al. Recommendations to address the difficulties encountered when determining linezolid resistance from whole-genome sequencing data. Antimicrob Agents Chemother 2018; 62: e00613-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mendes RE, Deshpande LM, Farrell DJ. et al. Assessment of linezolid resistance mechanisms among Staphylococcus epidermidis causing bacteraemia in Rome, Italy. J Antimicrob Chemother 2010; 65: 2329–35. [DOI] [PubMed] [Google Scholar]
- 31. Roman F, Roldan C, Trincado P. et al. Detection of linezolid-resistant Staphylococcus aureus with 23S rRNA and novel L4 riboprotein mutations in a cystic fibrosis patient in Spain. Antimicrob Agents Chemother 2013; 57: 2428–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hansen LH, Vester B.. A cfr-like gene from Clostridium difficile confers multiple antibiotic resistance by the same mechanism as the cfr gene. Antimicrob Agents Chemother 2015; 59: 5841–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vester B. The cfr and cfr-like multiple resistance genes. Res Microbiol 2018; 169: 61–6. [DOI] [PubMed] [Google Scholar]
- 34. Klupp EM, Both A, Belmar Campos C. et al. Tedizolid susceptibility in linezolid- and vancomycin-resistant Enterococcus faecium isolates. Eur J Clin Microbiol Infect Dis 2016; 35: 1957–61. [DOI] [PubMed] [Google Scholar]
- 35. Chacko KI, Sullivan MJ, Beckford C. et al. Genetic basis of emerging vancomycin, linezolid, and daptomycin heteroresistance in a case of persistent Enterococcus faecium bacteremia. Antimicrob Agents Chemother 2018; 62: e02007-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bourgeois-Nicolaos N, Massias L, Couson B. et al. Dose dependence of emergence of resistance to linezolid in Enterococcus faecalis in vivo. J Infect Dis 2007; 195: 1480–8. [DOI] [PubMed] [Google Scholar]
- 37. He T, Shen Y, Schwarz S. et al. Genetic environment of the transferable oxazolidinone/phenicol resistance gene optrA in Enterococcus faecalis isolates of human and animal origin. J Antimicrob Chemother 2016; 71: 1466–73. [DOI] [PubMed] [Google Scholar]
- 38. Toh SM, Xiong L, Arias CA. et al. Acquisition of a natural resistance gene renders a clinical strain of methicillin-resistant Staphylococcus aureus resistant to the synthetic antibiotic linezolid. Mol Microbiol 2007; 64: 1506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhanel GG, Love R, Adam H. et al. Tedizolid: a novel oxazolidinone with potent activity against multidrug-resistant Gram-positive pathogens. Drugs 2015; 75: 253–70. [DOI] [PubMed] [Google Scholar]
- 40. Bensaci M, Flanagan S, Sandison T.. Determination of Tedizolid susceptibility interpretive criteria for gram-positive pathogens according to clinical and laboratory standards institute guidelines. Diagn Microbiol Infect Dis 2018; 90: 214–20. [DOI] [PubMed] [Google Scholar]
- 41. Diaz L, Kiratisin P, Mendes RE. et al. Transferable plasmid-mediated resistance to linezolid due to cfr in a human clinical isolate of Enterococcus faecalis. Antimicrob Agents Chemother 2012; 56: 3917–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Inkster T, Coia J, Meunier D. et al. First outbreak of colonization by linezolid- and glycopeptide-resistant Enterococcus faecium harbouring the cfr gene in a UK nephrology unit. J Hosp Infect 2017; 97: 397–402. [DOI] [PubMed] [Google Scholar]
- 43. Bender JK, Fleige C, Klare I. et al. Detection of a cfr(B) variant in German Enterococcus faecium clinical isolates and the impact on linezolid resistance in Enterococcus spp. PLoS One 2016; 11: e0167042.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Candela T, Marvaud JC, Nguyen TK. et al. A cfr-like gene cfr(C) conferring linezolid resistance is common in Clostridium difficile. Int J Antimicrob Agents 2017; 50: 496–500. [DOI] [PubMed] [Google Scholar]
- 45. Lazaris A, Coleman DC, Kearns AM. et al. Novel multiresistance cfr plasmids in linezolid-resistant methicillin-resistant Staphylococcus epidermidis and vancomycin-resistant Enterococcus faecium (VRE) from a hospital outbreak: co-location of cfr and optrA in VRE. J Antimicrob Chemother 2017; 72: 3252–7. [DOI] [PubMed] [Google Scholar]
- 46. Schwarz S, Wang Y.. Nomenclature and functionality of the so-called cfr gene from Clostridium difficile. Antimicrob Agents Chemother 2015; 59: 2476–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Singh KV, Arias CA, Murray BE.. Efficacy of tedizolid against enterococci and staphylococci, including cfr+ strains, in a mouse peritonitis model. Antimicrob Agents Chemother 2019; 63: e02627-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Savini V, Gherardi G, Astolfi D. et al. Insights into airway infections by enterococci: a review. Recent Pat Antiinfect Drug Discov 2012; 7: 36–44. [DOI] [PubMed] [Google Scholar]
- 49. Silverman JA, Mortin LI, Vanpraagh AD. et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis 2005; 191: 2149–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All genomes sequenced in this study have been uploaded to the NCBI WGS database associated with BioProject PRJNA517335.