Abstract
Computed Tomography (CT) is the mainstay of diagnostic imaging evaluation of thoracic disorders. However, there are a number of CT protocols ranging from a simple non-contrast CT at one end of the spectrum, and CT perfusion as a complex protocol available only on high-end scanners. With the growing diversity, there is a pressing need for radiologists, and clinicians to have a basic understanding of the recommended CT examinations for individual indications. This brief review aims to summarise the currently prevalent CT examination protocols, including their recommended indications, as well as technical specifications for performing them.
Keywords: Computed tomography angiography, computed tomography chest, computed tomography contrast, dual energy computed tomography, low-dose computed tomography, protocol, pulmonary embolism
Introduction
Computed Tomography (CT) forms the workhorse of imaging evaluation of thoracic diseases. With the ever-expanding clinical spectrum of thoracic disorders, and diseases with thoracic manifestations, there has been increasing frequency of chest CT being ordered. Hence, there is a growing need for the radiologist, and the referring clinician to have a basic understanding of the recommended CT examination for specific individual indications.
The attendant concern of increased radiation exposure, especially in young individuals, as well as the use of iodinated intravenous contrast material in contrast-enhanced CT should be kept in mind while ordering such investigations, more so, in patients who require repeated imaging. Hence, a balance between attaining adequate diagnostic information and patient safety needs to be kept in perspective. In another clinical scenario, where patients may move across institutions or hospitals for treatment, with the availability of standardised imaging protocols, there should be no need for repeat imaging as recurrent imaging would entail additional radiation burden and contrast administration.
Although ultrasound and magnetic resonance imaging (MRI) are also being increasingly used in the evaluation of the chest, this review however, confines itself to CT. This review article aims to summarize the recommended thoracic CT protocols for various clinical/radiological indications including their technical parameters as well as applications of newer advancements in day-to-day clinical scenario based on current literature. The review does not address the status of ECG-gated cardiac/aortic CT. This manuscript was prepared as a part of initiative of Indian College of Radiology and Imaging (ICRI) to standardise imaging protocols in chest imaging.
Protocols vs Clinico-Radiographic Indications
The indication to perform a chest CT can be clinical, or based on an abnormality on prior imaging studies such as chest radiograph (CXR), fluoroscopy, MRI or abdomen CT. In almost all clinical scenarios, chest radiograph should be performed initially. Findings on CXR would help to tailor the appropriate CT examination. However, in several instances, CT will be performed even if the chest radiograph is normal e.g. Pyrexia of unknown origin (PUO). Hence, the use of the term clinico-radiographic indications is apt.
Broadly, the protocols may be divided into non-contrast CT (NCCT), contrast-enhanced CT (CECT) and then multiple modifications are available within this basic framework [Table 1]. Table 2 details the choice of CT protocol based on the clinico-radiographic indications. There are certain aspects of CT acquisition, though, which are common amongst all the protocols. Currently, all thoracic CT scans should be acquired in volumetric mode in full inspiration. Sequential mode may be used while acquiring limited expiratory scans. Certain clinical situations mandate the use of intravenous (IV) contrast such as evaluation of pyrexia of unknown origin (PUO) or acute infections. Omission of contrast in such scenarios can lead to inadequate evaluation of critical findings like mediastinal lymph nodes or complications.
Table 1.
Types of Imaging protocols
| CT protocols | |
|---|---|
| Non-contrast CT chest protocols | Routine NCCT |
| LDCT | |
| Ultra -LDCT | |
| Cine CT | |
| Contrast-enhanced CT chest protocols | Routine CECT |
| CT angiography protocols | CTPA CTA Combined protocols Split-bolus protocol Triple rule out |
| For masses/nodules (Functional Imaging) | Dynamic multiphasic CT Perfusion CT |
NCCT - Non-contrast CT; LDCT - low dose CT; CECT - Contrast enhanced CT; CTPA -CT pulmonary angiography; CTA - CT angiography
Table 2.
Choice of Chest CT protocol according to clinico-radiographic scenario
| Clinicoradiographic indications | CT Protocol |
|---|---|
| Screening for pulmonary metastasis | NCCT |
| As part of Lung Cancer Screening Program | LDCT |
| Unexplained dyspnoea on exertion, suspected or known case of ILD (e.g. In connective tissue diseases, rheumatological disorders), follow -up of ILD | NCCT |
| Suspected or known bronchiectasis, small airway disease | NCCT/LDCT |
| PUO | CECT (chest and abdomen) |
| Non-resolving pneumonia | CECT |
| Mediastinal widening | CECT |
| Malignant pleural effusion, empyema, chest wall disease | CECT |
| Staging and follow-up of lung cancer | CECT, Biphasic protocols* |
| Staging and follow -up of lymphoma | CECT (neck, chest and abdomen) |
| Unexplained vocal cord palsy | CECT |
| Evaluation of solitary pulmonary nodules | CECT, Dynamic multiphase CT |
| Blunt or penetrating chest trauma | CECT, CTA |
| Recurrent/Significant haemoptysis | CTA, Split-bolus CTA |
| Atypical chest pain (additional diagnosis other than acute coronary syndrome is considered e.g., Pulmonary embolism or aortic dissection) | CTA |
| Intermediate to high clinical probability of pulmonary thromboembolism or positive D-dimer level including pregnant patients (ACR Appropriateness Criteria, revised 2016) |
CTPA |
NCCT- refers to routine non contrast CT as detailed in the text; ILD- interstitial lung disease ;CECT - Contrast enhanced CT; CTPA- CT pulmonary angiography; CTA - CT angiography; *. Is used when these are to be used in special situations detailed in text
Non-Contrast CT (NCCT) Chest Protocols
NCCT chest can be performed in various protocols such as routine NCCT in standard dose, low dose CT (LDCT) or Ultra-LDCT and their indications and technical parameters are summarized in Table 3.
Table 3.
NCCT chest protocols according to indications
| Routine NCCT/standard dose CT | Low-dose CT | Ultra Low-Dose CT | |
|---|---|---|---|
| Indications | Suspected pulmonary metastases ILD Bronchiectasis Deranged renal parameters Febrile neutropenia |
ILD evaluation* Lung cancer screening Follow-up imaging for nodules/pulmonary infections, bronchiectasis, ABPA, CF |
Lung cancer screening/nodule detection Screening lung as a part of coronary CTs. |
| Acquisition protocol | |||
| kVp | 100 (<~80kg) 120 (~80-113kg) 140 (>113kg) |
80-120 | 120-140 |
| mA with AEC | 35 | 22 | Without AEC |
| Tube current (mAs) | 130-200 | 20-40 | Employs a fixed mAs value- 10 |
| Collimation | Lowest possible on the scanner (e.g. 0.6) | Lowest possible on the scanner | Lowest possible on the scanner |
| Pitch | 1.2 | Highest pitch | NS |
| Rotation time | 0.5 | 0.5 | NS |
| Slice thickness | 1.2-1.5mm | 1.2-1.5mm | NS |
| Matrix | 512×512 | 512×512 | 512 × 512 |
| Expected Radiation Dose | 3-8 mSv# | 1-3 mSv | 0.182±0.028mSv i.e. <1 mSv |
*Use of LDCT in ILD is recent concept, explained in text.; ABPA- Allergic bronchopulmonary aspergillosis; CF- Cystic fibrosis; ILD - interstitial lung diseases; NS- Non specified. #With modern scanners and availability of iterative reconstructions, a radiation dose of 2-3mSv is possible
Routine NCCT
Frequent indications of NCCT chest are suspected cases or follow up of metastasis, bronchiectasis, ILD and pulmonary infections.
Image acquisition
All non-contrast scans should be acquired in volumetric mode, scanning extending from thoracic inlet to caudally include upper abdomen [Figure 1]. Patients are imaged in supine position in suspended deep inspiration with arms extended overhead to reduce beam hardening artefact. Some modifications of the NCCT scans include expiratory and prone scans.
Figure 1 (A-D).
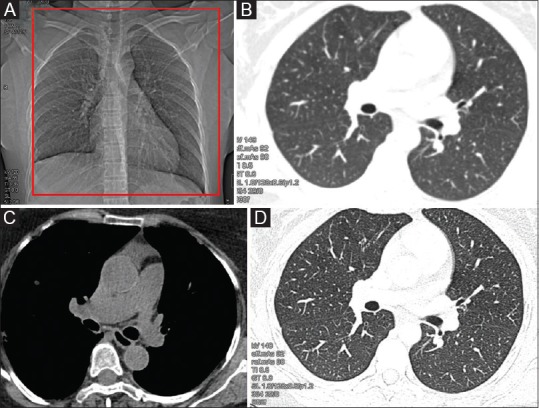
NCCT Chest (Routine): (A) Supine topogram image: the red box shows the area of coverage for routine chest CT, extending from thoracic inlet to upper abdomen. Images reconstructed in soft kernel in standard lung b50s (B) and mediastinal windows b30s (C); and in high-resolution reconstruction algorithm b80s (D)
Image reconstructions
The acquired CT images are reconstructed into soft tissue mediastinal window (20-30 kernel) and lung window (in sharp algorithm, 60-80 kernel) and in 1.2 -1.5 mm section thickness for interpretations. The 60-80 kernel reconstruction becomes the high resolution CT and hence HRCT chest need not be acquired in sequence mode separately as in earlier times. Thus, non-volumetric sequential mode HRCT has no indication in current era and any volumetric CT chest data can be reconstructed in high-resolution algorithm. The classic HRCT chest protocol was used in earlier times for acquiring high resolution images of lung parenchyma. The technique includes using thin collimation, usually 1-2 mm, which improves the spatial resolution and coupled with a high spatial frequency reconstruction algorithm which makes the structure visibly sharper. The images are acquired at 10mm interval, thereby considerably reducing the radiation dose. In the centres where MDCT is not available, HRCT acquisition is still used for evaluation of diffuse lung diseases.
The volumetric data can be further subjected to various post-processing on workstations such as multiplanar reconstructions (MPRs), maximum intensity projection (MIP), minimum intensity projection (minIP), volume rendered images for virtual bronchography, and virtual bronchoscopy to evaluate airways [Figure 2]. Amongst these, minIP should be performed whenever airways are to be evaluated, and MIP images for nodule identification.
Figure 2 (A-D).
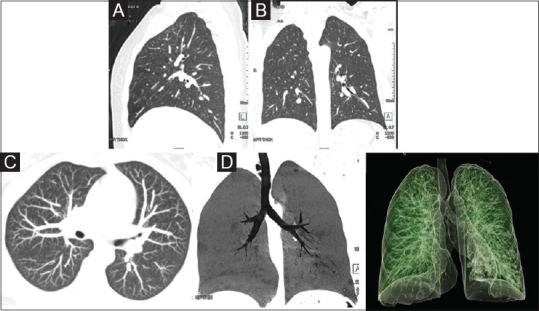
Image reformatting: (A and B) Sagittal and coronal reformation in standard lung window to allow three-dimensional view. (C) Axial maximum intensity projection (MIP) in lung window for enhanced visualization of nodules. (D) Coronal minimum intensity projection (minIP) in lung window for assessment of tracheobronchial airway. (D) Virtual bronchography (volume rendered display) for visualization of airways
Expiratory scan
These scans are mainly acquired in cases of suspected air-trapping, small airway disease or tracheobronchomalacia, and may include contiguous acquisition similar to the supine inspiratory scan, or a sequential three-step acquisition involving upper thoracic trachea, carina and lung bases through the diaphragm [Figure 3]. Scans can be acquired as sequential step-wise acquisition which delivers less radiation and is preferred in young patients, while volumetric mode can be used in elderly patients. Whenever there is appearance of ground glass opacities, mosaic pattern on routine NCCT or suspicion of an airway dominant disease, it is necessary to acquire an expiratory CT. In fact a further modification of this is the cine CT described subsequently in detail.
Figure 3 (A and B).
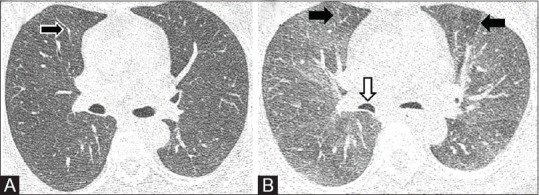
NCCT variants. (A) Inspiratory scan showing mild mosaic attenuation (arrow). (B) Expiratory CT performed in low dose shows multiple areas of air trapping (black arrows). Note the bowing of posterior walls of bronchi suggesting adequate expiratory phase (white arrow)
Prone scans
Prone position is advised to differentiate between dependent density and features of early interstitial lung disease, when there is equivocal increase in attenuation and reticulation noted on dependent parts of bilateral lung parenchyma on routine supine scan [Figure 4]. The acquisition should be restricted to the area of interest.
Figure 4 (A and B).
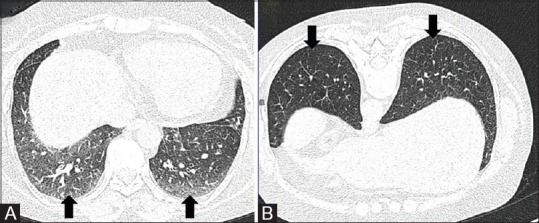
NCCT variants. NCCT chest in supine (A) position shows ground glass opacities in posterior basal segments of bilateral lower lobes (arrows). CT performed in prone position (B) shows clearing of the GGOs suggestive of dependent densities (arrows)
Low-dose CT (LDCT)
LDCT protocols have been developed for patients requiring repeated imaging such as several patients with interstitial lung disease (ILD) or nodule. Hence, the need to adhere to ALARA (As Low As Reasonably Achievable) principle cannot be over emphasized. Guidelines exist for dose optimization depending on indications.
For nodule detection/Lung cancer screening
Low-dose protocol is recommended for pulmonary nodule follow-up as well as screening for lung cancer in select high-risk patients.[1] Low-dose CT refers to scanning techniques which use tube current less than 100 mAs in an attempt to deliver reduced radiation dose to the patient while maintaining diagnostic quality images.[2] However, several technical parameters relating to the scan acquisition needs to be modified to achieve optimal results. Usually, low dose CT is performed using tube voltage of 120 kVp (KVp is adjusted according to patient's weight) and tube current ranging from 40-80 mAs. Such low tube-current generates increased image noise, which can be partially compensated by using iterative reconstruction algorithm (now available with many CT manufacturers). However, the vast majority of scanners in the world are still without iterative reconstructions software and use images reconstructed using conventional filtered back-projection [Figure 5].
Figure 5 (A-C).
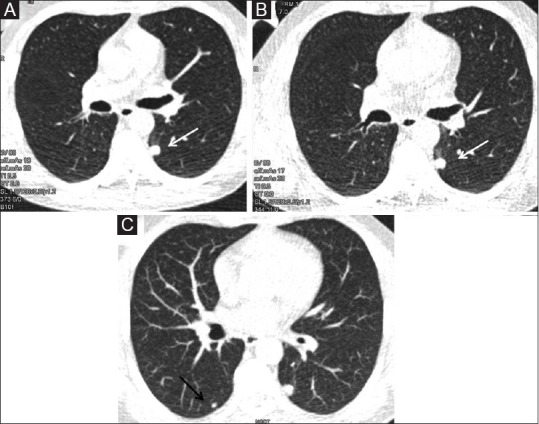
Low dose CT (LDCT) nodule follow-up. (A). LDCT done in a 46-year old chronic smoker shows a nodule in left lower lobe (white arrow).(B) Follow-up LDCT done 6 months later shows no interval change in size. (C) Axial maximum intensity projection (MIP) image in lung window shows another smaller nodule (black arrow) in right lower lobe in addition to left lower lobe nodule
Reconstructed slice thickness is kept at 2.5 mm or higher (for non-targeted FOV in low frequency algorithm) while a slice thickness of 1.0-1.5 mm at high frequency algorithmic reconstruction needs to be done through the region of interest (ROI) to improve spatial resolution. The ROI needs to be viewed in both low- and high-spatial-frequency algorithm as the latter reconstruction may erroneously show high density edge artifacts simulating calcium. Recent evidence shows that there is no significant difference in inter-observer and intra-observer variability in measurement of nodule volumes when low-dose CT is compared to standard dose CT, suggesting that the former can be used for follow up of pulmonary nodules.[2,3,4,5] Various studies have been performed with different combinations of kVp and mAs in the context of low-dose CT leading to variable estimated doses, but on an average an acceptable low-dose CT screening can be performed with an approximate effective dose of 2mSv.[6]
For suspected ILD
Use of low dose CT is a recent concept in imaging of ILD. An attempt should be made henceforth to modify unstructured protocols and achieve dose <3 mSv even in ILD patients. The current recommendations for performing CT for imaging evaluation of suspected ILD includes volumetric acquisition with sub-millimetric collimation, highest pitch and shortest rotation time. Tube voltage and tube current are determined based on patient size. The recommended acquisitions include supine sustained end-inspiratory phase (volumetric) and supine sustained end-expiratory (volumetric or sequential) phase [Figure 6]. Additional optional scan includes prone inspiratory scan (volumetric or sequential) limited to lower lobes. Image reconstruction is done at thin slices (1.5 mm) at high-spatial-frequency algorithm which can be contiguous or overlapping. Post-processing tools including minIP and MIP to be used to assess low attenuation lesions and micronodular infiltration, respectively. The radiation dose for first acquisition (i.e. volumetric inspiratory) should be between 1 to 3 mSv (low-dose CT), with added caution to avoid ultra-low dose CT (<1 mSv).[7]
Figure 6 (A-D).
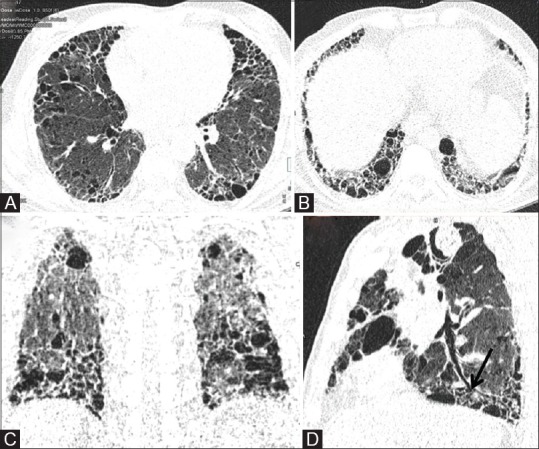
LDCT for evaluation of ILD. (A) Axial image of LDCT in high resolution reconstruction algorithm in a 65-year old chronic smoker with suspected ILD. (B and C) Routine axial and coronal reconstruction in lung window allow evaluation of cranio-caudal and axial distribution of reticulations, interlobular septal thickening and macrocysts. (D) Curved MPR along the axis of bronchus (black arrow) allows distinction between honeycombing or macrocysts and traction bronchiolectasis
Ultra-low dose CT
When doses are kept at <1 mSv, it is often referred to as ultra-low dose CT. Its utility has been shown in screening for lung cancer and in screening for lung nodules as a part of coronary CT.[8] This is strictly restricted to screening/surveillance and not for routine clinical evaluation of lung parenchyma.
Cine CT
Cine CT is not available in every scanner, and if available tailor coverage to airway (critical areas being lower trachea and carina). Cine CT without table feed during free breathing has been investigated as an alternative diagnostic modality for assessment of dynamic airway collapsibility and has been found to have comparable diagnostic efficacy as flexible bronchoscopy, which is considered the gold standard for diagnosis[9,10,11] [Figure 7]. In fact, the former is considered better than paired static inspiratory/expiratory images. Initial studies had used electron beam CT and single detector CT for cine acquisition of airway during functional manoeuvres and showed encouraging results, albeit with limited z-axis coverage and increased radiation dose.[12] Subsequently, 64-slice MDCT with z-axis coverage of more than 3 cm was successfully employed for dynamic airway assessment during coughing.[13] The anatomical coverage was further extended to 16 cm using a 320-row MDCT by Wagnetz U et al. to cover the airway from lower end of larynx to the origin of lower lobe bronchi.[14]
Figure 7 (A and B).
Cine CT showing dynamic collapsibility of right main bronchus more than 50 percent suggestive of bronchomalacia. (A) Saved screenshot of inspiratory phase. (B) Saved screenshot of expiratory phase. (C)Video file (supplementary).
Recently, respiratory gated 4D CT of the whole chest has been performed with a dose-modulated protocol at a pitch of 0.09 (lowest possible), minimal exposure parameters (80 kVp and 10 reference mAs) and thin collimation (16 × 1.2 mm). Image reconstruction was done using iterative reconstruction algorithm, at slice thickness of 1.5mm at increments of 1.0 mm in a medium soft kernel.[15] The reported radiation dose ranged from 2.9 to 3.1 mGy CTDIvol. The radiology technicians should be aware of such advanced protocols available in the scanners.
Contrast Enhanced CT (CECT) Chest Protocols
The numerous options of contrast enhanced scans range from a routine contrast enhanced CT (CECT) to CT perfusion scans. Various contrast media are available for use in contrast enhanced CTs. For routine CECT, iodine concentration of 300-350 mgI/ml suffices whereas for CT angiography protocols, a higher concentration of 350-400 mgI/ml is preferred. The dose of contrast to be administered depends on the weight of the patient and current CT scanners allow 1-1.5 ml/kg to be used for routine CECT as well as CTA protocols. Doses up to 2 ml/kg may sometimes be employed for CTA protocols in obese patients. Availability of Dual energy CT scanners further helps to reduce the contrast dose because of better contrast visualization at lower keV images.
Routine CECT chest
Routine CECT chest essentially retains the same parameters as NCCT with administration of intravenous contrast. Usually a scan delay of 55-70 seconds is kept following administration of contrast to allow for optimal enhancement of soft tissues[16] [Figure 8]. Contrast is usually delivered using hand injection with optional use of power injector and bolus chase. CECT chest is often coupled with upper abdomen (liver and adrenals) for baseline staging and follow-up of lung cancer, and with abdomen and neck for PUO evaluation or other malignancies such as lymphoma staging. Two different protocols exist for the same: the first one involves single delayed acquisition of chest and abdomen. The other one uses dual acquisition-an earlier acquisition for chest (20-35 seconds delay), and a second delayed acquisition for abdomen in portal venous phase. Although, no consensus exists over the superiority of one protocol over another, recent evidence suggests that a single 60 seconds delayed scan covering chest and abdomen offers better image quality in terms of improved lymph nodal visualisation, reduced perivenous contrast- related artefacts, lower radiation dose with acceptable thoracic vascular and hepatic parenchymal enhancement.[17] Some authors also suggest a modified contrast infusion protocol for better visualisation and characterisation of a pleural disease with a greater infusion rate (150 ml at 2.5 ml/sec).[18]
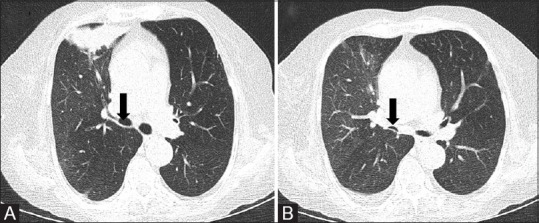
Figure 8 (A-C).
(A-C) Contrast-enhanced CT chest (Routine). Axial, coronal and sagittal reformations in standard mediastinal window. The cross-bar allows three-dimensional localization of lesion
The various modifications of contrast-enhanced scans as discussed below and detailed in Table 4 are related to the volume, rate and manner of contrast injection (monophasic vs biphasic), timing of scan acquisition and use of dual energy.
Table 4.
Protocols of contrast enhanced scans for opacification of pulmonary and systemic circulation
| CTPA (Pulmonary circulation) | CTA (Systemic Circulation) | Combined protocol- (Pulmonary + systemic circulation) | |
|---|---|---|---|
| Indication | Pulmonary embolism | Aorta and its branches | Both pulmonary artery and aorta and its branches. |
| Area of coverage | Lung apex to level of diaphragm in all cases. Just above aortic arch to just below heart (in young or pregnant patients with normal CXR and minimal suspicion of PE) | Thoracic inlet to lower border of L2 | Thoracic inlet to lower border of L2 |
| Slice thickness For reconstruction | ≤2mm | ≤1.5mm | ≤1.5mm |
| kVp | For patients <30 years and 30-60 years: 120, 100 or 80 kVp depending on patient BMI or automatic tube voltage selection For patients >60 years: 120 kVp | 100kVp | Dual energy (80kVp and 140kVp |
| mAs | For patients <30 years: 150 mA with tube current modulation. For patients 30-60 years and >60 years: 200 mA with tube current modulation. | Automated tube current modulation | Automated tube current modulation |
| Rate of contrast injection (where applicable) | 3-4ml/s (normal resting heart rate) 4-5ml/s (elevated cardiac output) | 3.5-4ml/sec | 3/4th contrast at 5ml/s 1/4th contrast at 3ml/s Saline chase at 3ml/s |
| ROI position (where applicable) | Pulmonary trunk or MPA or right atrium | Descending aorta | Ascending aorta |
| Threshold HU (where applicable) | 60-100HU* | 100HU | 100HU |
| Time delay/phase of acquisition | 4-5 sec after bolus trigger time | 6 sec after bolus trigger time | 5 sec after bolus trigger time |
| Reconstruction | Contiguous image reconstruction at intermediate-spatial-resolution algorithm. View in cine mode at window width 450-600HU and window level 35-100HU (#) and maximum intensity projections (MIP) |
Reconstruction interval 2.0mm or similar depending upon scanner, at standard mediastinal window (Window level 40HU, window width 400HU) | Axial image reconstruction at 1.5 mm slice thickness and increment of 1.2 mm at standard mediastinal and lung windows. |
*60 HU is used by several dual source DECT scanners. #Pulmonary embolism can be missed when images with very bright contrast is viewed only on mediastinal window settings
Protocols for primary visualisation of vascular tree (CT angiography protocols)
Different CT angiography protocols are devised to account for different time of peak opacification of the two vascular trees of chest, pulmonary and systemic (aortic) following IV contrast, with the former opacifiying 10-14 seconds earlier.
CT Pulmonary angiography (CTPA)
CTPA is performed for suspected pulmonary embolism (PE), follow up of PE and evaluation of cause of pulmonary artery hypertension. CTPA should be performed during shallow inspiratory breath hold; mainly to avoid Valsalva manoeuvre associated with deep inspiration during the acquisition as it can lead to poor vascular opacification due to dilution of contrast bolus with unopacified blood flowing from inferior vena cava.[19] For very sick patients, or those who cannot follow respiratory commands, CTPA can also be performed with free-breathing by increasing the pitch in dual-source CT scanners (up to 3) so that the scan is complete in less than a second.[20] The same when coupled with low-voltage and iterative reconstruction algorithm can substantially reduce the radiation dose with acceptable image quality.[19]
CTPA scans should be acquired in caudo-cranial direction to avoid streak artefacts from dense contrast media in superior vena cava or subclavian vein, thus allowing time for the saline chaser to clear off the contrast to chest by the time imaging proceeds to that level [Figure 9]. To avoid these artefacts, some authors have also proposed a triphasic contrast injection protocol that allows for graded decrease in contrast concentration in a phasic manner i.e., Phase 1: 50 ml of undiluted contrast agent, phase 2: 30 ml of contrast in 70%:30% dilution of saline and contrast, and phase 3: 50 ml of pure saline).[20] A minimum mean attenuation value of 250 HU is required in main pulmonary artery for accurate diagnosis of pulmonary embolism.[21]
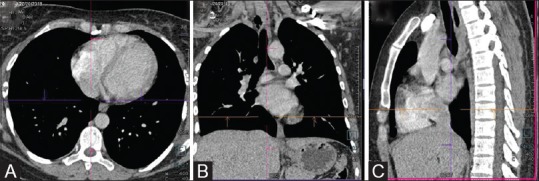
Figure 9 (A-D).
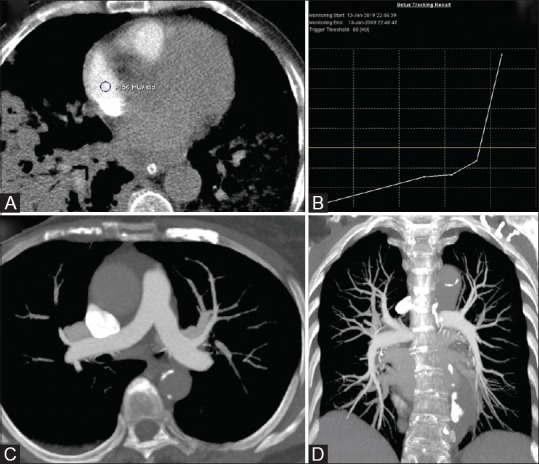
CT Pulmonary angiography (CTPA on a DECT scanner). (A) ROI positioning in right atrium. (B) Enhancement curve for bolus tracking showing the beginning of acquisition at 60HU. Axial (C) and coronal (D) MIP images show the optimal visualisation of main pulmonary artery and peripheral branches
With the advent of dual-energy CT, there have been several advancements in the technical aspect of image acquisition and post-processing of CTPA. The most important advantages of image acquisition in dual energy mode are: (1) generation of material density display which can be used to generate iodine perfusion maps providing panoramic visual assessment of lung parenchyma thereby, highlighting any focal areas of perfusion defect, (2) generation of virtual non-contrast images, and (3) post-processing for image optimization (like low keV images can be used even if the contrast opacification was not adequate due to an error in timing or calculating the dose of contrast bolus).[19] Several studies have shown that a combination of high pitch (using dual source scanner), low tube-voltage acquisition with iterative reconstruction for post-processing can not only significantly reduce patient dose with comparable image quality, but can also achieve improved temporal resolution (reducing motion artefacts and allowing simultaneous cardiovascular evaluation) with reduced contrast dose.[19]
In cases of chronic pulmonary thromboembolism (PTE), the CT can be done in two phases (pulmonary and systemic) when using dual energy CT. This helps to generate iodine perfusion maps, to demonstrate the areas of perfusion defects in pulmonary arterial phase, which subsequently gets opacified in late (aortic) phase if sufficient collateralisation has taken place[22] [Figure 10]. Even when using single source MDCT scanners, biphasic protocol is advocated for demonstration of systemic collaterals that develop in chronic PTE.
Figure 10 (A-D).
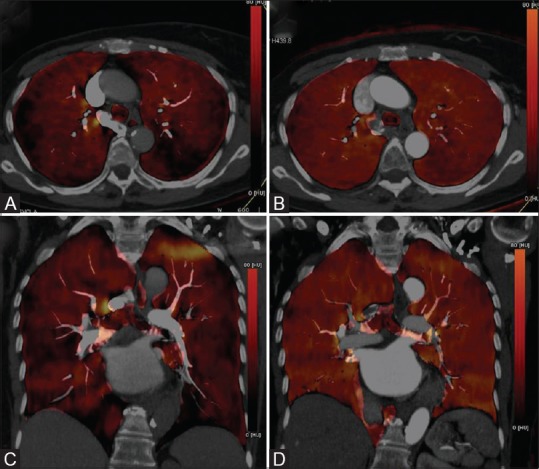
Iodine map (DECT-CTPA) in an 83-year old female with chronic pulmonary thromboembolism. (A and C) Axial and coronal color-coded iodine map overlay generated from pulmonary arterial phase shows multiple wedge-shaped perfusion defects peripherally in bilateral hemithorax. (B and D) Axial and coronal colour –coded iodine map overlay of subsequently acquired aortic phase; shows uniform distribution of iodine bilaterally suggestive of homogenous arterial perfusion (through systemic collaterals), filling previous areas of pulmonary perfusion defects
CT Angiography for systemic arteries
The term CT angiography (CTA) is used when primarily the focus is on systemic circulation (aorta and its branches) [Figure 11]. CTA of thorax is essentially performed in three scenarios 1. Evaluation of hemoptysis which includes pre-procedural imaging for bronchial artery embolization, 2. Suspected aortic dissection/aneurysm, 3. Demonstration of anomalous artery in congenital lung malformations (e.g., sequestration). Among these, CTA for aortic dissection/aneurysm evaluation requires an ECG gated acquisition, the complete discussion of which is beyond the scope of this article.
Figure 11 (A-D).
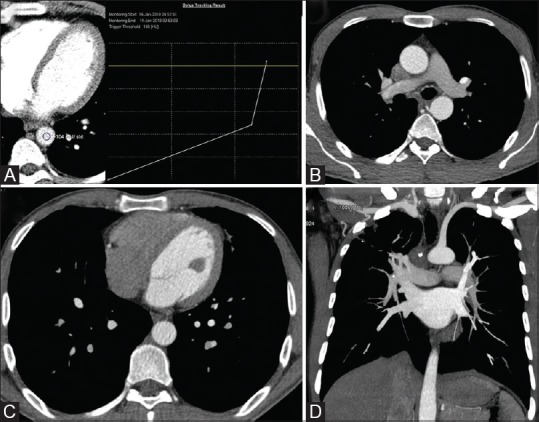
CT thoracic angiography - CTA. (A) ROI positioning in descending thoracic aorta with trigger at 100 HU. Axial (B and C) and Coronal (D) reconstructed images show faint attenuation of right sided heart and pulmonary arteries, with predominant opacification of left sided heart and aorta
CT angiography for haemoptysis
MDCT angiography of thorax is increasingly being recommended in the diagnostic work-up and management of hemoptysis, especially for planning of bronchial artery embolization (BAE).[23] Antoine Khalil et al.[24] described an angiographic protocol on 16-slice scanner (120kV and 180 mA) which involved cranio-caudal scan acquisition from lung apices to lung bases in supine position and maximum inspiratory breath hold. However, a more extensive coverage from the base of the neck till the level of renal arteries is recommended, corresponding to L2 vertebral level to include non-bronchial systemic supply from branches of arch of aorta and infra-diaphragmatic arteries.[25] The protocol is described in Table 4.
Combined protocols (For pulmonary and systemic circulation)
There is a growing trend towards achieving adequate opacification of both aortic and pulmonary systems in a single acquisition to allow simultaneous evaluation without additional exposure of multiple scans. This is primarily achieved by using split-bolus techniques for contrast administration or prolonged administration of contrast.
Split-bolus protocol for hemoptysis
A comprehensive evaluation into the source of hemoptysis requires optimal enhancement of both bronchial (systemic) and pulmonary arterial bed in a single examination.[26] Hence, CT using biphasic contrast bolus injection is advocated for optimal visualization of both pulmonary arteries and thoracic aorta without additional radiation or contrast compared to monophasic contrast injection.[27] Although the earlier protocols used higher doses of undiluted contrast, the contrast dose has significantly come down with the current generation of scanners, especially with the introduction of dual energy CT. The authors have adopted a similar biphasic contrast-injection protocol (split-bolus) with added modifications, which is currently being followed at our institute for evaluation of haemoptysis and is described in Table 4. The scan thus acquired allows evaluation of bronchial arteries (branches of thoracic aorta), non-bronchial systemic supply as well as the pulmonary vessels, in a single acquisition with smaller contrast dose.[28] The benefit of opacification of pulmonary vessels is that in addition to detecting the pulmonary arterial causes of haemoptysis (like pseudoaneurysms), the dilated pulmonary arterial or venous branches (corresponding to areas of shunting) can also be well seen, aiding in exact localization of bleeding [Figure 12].
Figure 12 (A-D).
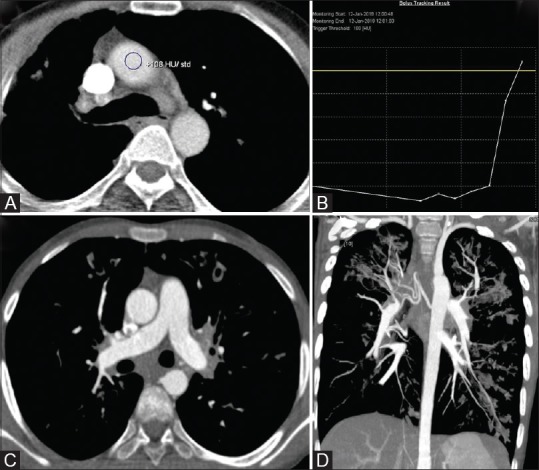
Single phase split-bolus DECT angiography in a case of hemoptysis. (A and B) ROI positioning in ascending aorta with trigger at 100HU. (C and D) Simultaneous optimal opacification of aorta, pulmonary artery and their branches is seen
Triple rule out CT (TRO CT)
TRO CT is a dedicated ECG gated examination aimed to evaluate aorta, coronary artery and pulmonary artery and mid-to lower thorax in a single scan. TRO is essentially used to rule out acute coronary syndrome (ACS), pulmonary embolism or acute aortic syndrome as the underlying cause for patients presenting with chest pain to emergency department (low-to moderate risk of ACS or non-ACS diagnosis considered).[29] The scanning length approximates 20 cm extending from above aortic arch till the caudal aspect of heart, and is acquired in less than 15 second under ECG gating. Scan parameters include tube voltage of 120kVp, mean effective tube current 600mAs per section with mean effective radiation dose in helical mode of scanning of 18mSv without tube current modulation and 8.75 mSv with modulation. There is extensive literature available on ECG gated triple rule-out CT, the details of which are beyond the scope of this article.
Protocols for multiphasic evaluation of masses/nodules (functional imaging)
Different tumours opacify at different timings following IV contrast injection based on the tumour histology and amount of angiogenesis. A single phase CT may hence fail to demonstrate the tumour and its extensions reliably. Also, nodules demonstrate different peak enhancement at different times depending on aetiology. Similarly, a biphasic acquisition in two phases maybe required if all arterial and venous anatomy needs to be analysed preoperatively or while evaluation of suspected hypervascular tumor in chest.
There are two basic modes of performing dynamic contrast-enhanced CT (DCE-CT): the more widely available and established technique uses multiple scan acquisitions at pre-determined time delays with respect to the initiation of contrast injection, while the newer ‘ first pass’ technique evaluates the initial passage of contrast media though the intravascular space by acquiring a baseline non-contrast image followed by a series of images after contrast administration. Analysis of the temporal changes in the contrast enhancement of the tissues allows generation of various perfusion-related parameters that reflect the vascular status of the tissue being studied.[30] The former more conventional approach to DCE-CT is routinely available on the current scanners and has more clinical acceptability while the latter is largely considered a research tool with limited availability.
Dynamic multiphase CT
The more conventional approach involves 5-6 images acquired through the nodule at fixed time intervals post contrast injection. This aids in characterization of the pulmonary nodules as benign or malignant, based on enhancement characteristics[31] [Figure 13]. A similar protocol has recently been adopted in SPUtnIK trial for characterization of incidental indeterminate solitary pulmonary nodules (SPN).[32] This mode of CT acquisition can be performed even on low-end scanners and the detector collimation varies accordingly. The suitability of the nodule for multiphase CT is initially assessed prior to performing multiphase CT. Hence, a baseline routine NCCT/LDCT is performed to confirm the size (atleast 8mm), visibility and soft tissue attenuation of nodule on mediastinal window. Tube voltage is kept at 100kVp for better contrast-to-noise ratio while the tube current is modulated according to patient size. Following injection of contrast, serial breath hold acquisitions are performed at 60 s, 120 s, 180 s and 240 s. Slice thickness is kept at 3 mm, with 2mm reconstruction interval. Nodule analysis is performed in mediastinal window (width: 400 HU, level: 40 HU) in axial plane. The analysis of resultant image set involves measurement of peak mean nodule enhancement and time-attenuation curve which can be performed on most of the routinely available commercial software.
Figure 13 (A-C).
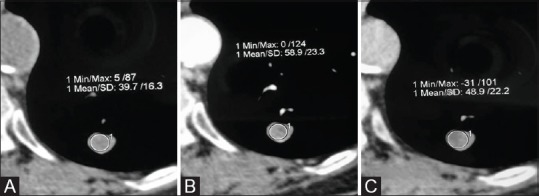
Multiphasic CT for evaluation of nodule enhancement. Magnified image in mediastinal window of a nodule in left lower lobe. (A) NCCT and images acquired at 30 secs (B) and 70 secs (C) post contrast injection, show awash-in of 19 HU
Perfusion CT/Dynamic ‘first pass’ CT
Dynamic first pass contrast–enhanced perfusion CT (DCE-CT) has been used by various investigators for quantitative assessment of perfusion parameters for characterization of pulmonary nodules or masses, as well as for early assessment of treatment response in lung cancer following chemotherapy.[33,34,35,36] The current role of DCE-CT is more as a complimentary modality to established investigations as CT and 18FFDG PET-CT for lesion characterization. The CT protocol outlined in the present review is based on the recommendations of on-going multi-centric SPUtNik trial which assesses the diagnostic performance, costs and health outcome of DCE-CT as an adjunct to 18FFDG PET-CT for nodule characterization. Prior to performing DCE-CT, it is essential to evaluate the suitability of the lesion using similar parameters as detailed in dynamic multiphasic protocol.[32]
The institutional protocol being followed at the authors’ centre for evaluation of perfusion in lung carcinoma uses dual-source DECT scanner with 50 ml of contrast (320 mg I/ml at 5 ml/second followed by 20 ml saline flush). Z-axis coverage varies from 7-10 cm depending on tumour. After a scan delay of 5 seconds, dynamic acquisition of 22 scans are performed in free breathing ( first 10 scans every 3 seconds, next 5 scans every 2 seconds and next 7 scans every 4 seconds). Tube voltage and tube current are kept at 80 kVp and 80mAs, respectively. Image reconstruction is done at slice thickness of 5mm, reconstruction interval of 3mm and mediastinal soft tissue kernel. Six perfusion parameters are analysed (peak enhancement intensity, blood flow, blood volume, permeability, mean transit time and time to peak) using two-compartment analysis model [Figure 14].[37] The Table 5 below summarises the key features of two modes of performing dynamic contrast enhanced CT for pulmonary nodules and masses.
Figure 14 (A-E).
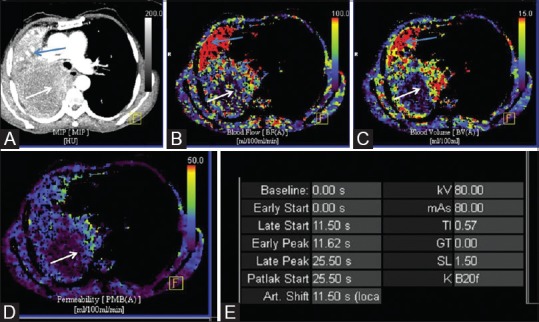
Dynamic perfusion CT of large necrotic mass to choose appropriate site of biopsy. (A) Axial maximum intensity projection (MIP) image in mediastinal window shows large necrotic mass in right hemithorax, with peripheral thick irregular nodular enhancement (white arrow) and associate collapse of lung (blue arrow). Color-coded blood flow (B), blood volume (C) and permeability (D) maps showing increased perfusion in peripheral nodular areas of enhancement (white arrow) with hypoperfused necrotic centre. Distally collapsed lung shows homogeneous high perfusion (blue arrow). (E) Image showing technical parameters of the acquisition
Table 5.
Key features of acquisition protocol of two subtypes of dynamic contrast-enhanced CT
| Dynamic multiphase CT | Dynamic ‘ first pass’/perfusion CT | |
|---|---|---|
| Area of coverage | 6 cm | 7-10 cm depending on tumour |
| Slice thickness | 3 mm | 5 mm |
| kVp | 100 kVp | 80 kVp |
| mAs | According to patient’s weight: <60 Kg, 200 mAs 60-90 Kg, 350 mAs >90kg. 500 mAs | 80 mAs |
| Rate of contrast | 2ml/sec | 5 ml/sec |
| Time delay/phase of acquisition | Pre-contrast, 60 sec, 120 sec, 180 sec and 240 sec. | Scan delay: 5 sec, 22 dynamic acquisitions: first 10 scans every 3 sec, next 5 scans every 2 sec and next 7 scans every 4 sec |
| Reconstruction | Reconstruction interval: 2mm Standard mediastinal window: (windowlevel: 40HU, width: 400HU) | Reconstruction interval: 3 mm Standard mediastinal window: (windowlevel: 40HU, width: 400HU) |
| Parameter available | Peak enhancement (HU), Wash in and wash out percentages | Blood flow, blood volume, permeability, mean transit time, time to peak |
Paediatric Application of CT Chest Protocols
While most of these protocols would be applicable to children, but typically multiphasic CT imaging should be restricted in children, and dynamic CT perfusion is almost never used for characterisation. Substitution with USG and MRI should be considered whenever feasible. Additional equipment and vendor specific features of dose reduction should be adopted, and general principles of ALARA e.g. restriction of coverage should be adhered to even more strictly. Elaborating on the specific paediatric protocols in terms of sedation, contrast doses, radiation concerns is beyond the scope of this article.
Conclusion
The use of CT for evaluation of thoracic disorders has increased manifold with the ever increasing clinical indications. Paralleling the recent developments in CT hardware and software, the various protocols of CT acquisition are also evolving with the main objective of reduced contrast and radiation dose, improved image quality and reduced scan time allowing for generation of evidence-based recommended CT protocols for specific indication. Hence, the need for radiologists and clinicians alike to be aware of the standardized thoracic CT protocols to allow for uniformity and consensus opinion across institutions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Video Available on: www.ijri.org
References
- 1.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gartenschläger M, Schweden F, Gast K, Westermeier T, Kauczor H, von Zitzewitz H, et al. Pulmonary nodules: Detection with low-dose vs conventional-dose spiral CT. Eur Radiol. 1998;8:609–14. doi: 10.1007/s003300050445. [DOI] [PubMed] [Google Scholar]
- 3.Hein PA, Romano VC, Rogalla P, Klessen C, Lembcke A, Bornemann L, et al. Variability of semiautomated lung nodule volumetry on ultralow-dose CT: Comparison with nodule volumetry on standard-dose CT. J Digit Imaging. 2010;23:8–17. doi: 10.1007/s10278-008-9157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christe A, Torrente JC, Lin M, Yen A, Hallett R, Roychoudhury K, et al. CT screening and follow-up of lung nodules: Effects of tube current-time setting and nodule size and density on detectability and of tube current-time setting on apparent size. AJR Am J Roentgenol. 2011;197:623–30. doi: 10.2214/AJR.10.5288. [DOI] [PubMed] [Google Scholar]
- 5.Karabulut N, Törü M, Gelebek V, Gülsün M, Ariyürek OM. Comparison of low-dose and standard-dose helical CT in the evaluation of pulmonary nodules. Eur Radiol. 2002;12:2764–9. doi: 10.1007/s00330-002-1368-4. [DOI] [PubMed] [Google Scholar]
- 6.Larke FJ, Kruger RL, Cagnon CH, Flynn MJ, McNitt-Gray MM, Wu X, et al. Estimated radiation dose associated with low-dose chest CT of average-size participants in the National Lung Screening Trial. AJR Am J Roentgenol. 2011;197:1165–9. doi: 10.2214/AJR.11.6533. [DOI] [PubMed] [Google Scholar]
- 7.Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198:e44–68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 8.Zanon M, Pacini GS, de Souza VVS, Marchiori E, Meirelles GS, Szarf G, et al. Early detection of lung cancer using ultra-low-dose computed tomography in coronary CT angiography scans among patients with suspected coronary heart disease. Lung Cancer. 2017;114:1–5. doi: 10.1016/j.lungcan.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Heussel CP, Hafner B, Lill J, Schreiber W, Thelen M, Kauczor HU. Paired inspiratory/expiratory spiral CT and continuous respiration cine CT in the diagnosis of tracheal instability. Eur Radiol. 2001;11:982–9. doi: 10.1007/s003300100818. [DOI] [PubMed] [Google Scholar]
- 10.Baroni RH, Feller-Kopman D, Nishino M, Hatabu H, Loring SH, Ernst A, et al. Tracheobronchomalacia: Comparison between end-expiratory and dynamic expiratory CT for evaluation of central airway collapse. Radiology. 2005;235:635–41. doi: 10.1148/radiol.2352040309. [DOI] [PubMed] [Google Scholar]
- 11.Lee EY, Litmanovich D, Boiselle PM. Multidetector CT evaluation of tracheobronchomalacia. Radiol Clin North Am. 2009;47:261–9. doi: 10.1016/j.rcl.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Hasegawa I, Feller-Kopman D, Boiselle PM. 2003 AUR memorial award. Dynamic expiratory volumetric CT imaging of the central airways: Comparison of standard-dose and low-dose techniques. Acad Radiol. 2003;10:719–24. doi: 10.1016/s1076-6332(03)80117-4. [DOI] [PubMed] [Google Scholar]
- 13.Boiselle PM, Lee KS, Lin S, Raptopoulos V. Cine CT during coughing for assessment of tracheomalacia: Preliminary experience with 64-MDCT. AJR Am J Roentgenol. 2006;187:W175–7. doi: 10.2214/AJR.05.1456. [DOI] [PubMed] [Google Scholar]
- 14.Wagnetz U, Roberts HC, Chung T, Patsios D, Chapman KR, Paul NS. Dynamic airway evaluation with volume CT: Initial experience. Can Assoc Radiol J. 2010;61:90–7. doi: 10.1016/j.carj.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Wielpütz MO, Eberhardt R, Puderbach M, Weinheimer O, Kauczor HU, Heussel CP. Simultaneous assessment of airway instability and respiratory dynamics with low-dose 4D-CT in chronic obstructive pulmonary disease: Atechnical note. Respiration. 2014;87:294–300. doi: 10.1159/000357448. [DOI] [PubMed] [Google Scholar]
- 16.Iezzi R, Larici AR, Franchi P, Marano R, Magarelli N, Posa A, et al. Tailoring protocols for chest CT applications: When and how? Diagn Interv Radiol. 2017;23:420–7. doi: 10.5152/dir.2017.16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García-Garrigós E, Arenas-Jiménez JJ, Sánchez-Payá J. Best protocol for combined contrast-enhanced thoracic and abdominal CT for lung cancer: A single-institution randomized crossover clinical trial. AJR Am J Roentgenol. 2018;210:1226–34. doi: 10.2214/AJR.17.19185. [DOI] [PubMed] [Google Scholar]
- 18.Raj V, Kirke R, Bankart MJ, Entwisle JJ. Multidetector CT imaging of pleura: Comparison of two contrast infusion protocols. Br J Radiol. 2011;84:796–9. doi: 10.1259/bjr/55980445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albrecht MH, Bickford MW, Nance JW, Zhang L, De Cecco CN, Wichmann JL, et al. State-of-the-art pulmonary CT angiography for acute pulmonary embolism. AJR Am J Roentgenol. 2017;208:495–504. doi: 10.2214/AJR.16.17202. [DOI] [PubMed] [Google Scholar]
- 20.Kerl JM, Bauer RW, Renker M, Weber E, Weisser P, Korkusuz H, et al. Triphasic contrast injection improves evaluation of dual energy lung perfusion in pulmonary CT angiography. Eur J Radiol. 2011;80:e483–7. doi: 10.1016/j.ejrad.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 21.Bae KT, Tao C, Gürel S, Hong C, Zhu F, Gebke TA, et al. Effect of patient weight and scanning duration on contrast enhancement during pulmonary multidetector CT angiography. Radiology. 2007;242:582–9. doi: 10.1148/radiol.2422052132. [DOI] [PubMed] [Google Scholar]
- 22.Hong YJ, Kim JY, Choe KO, Hur J, Lee HJ, Choi BW, et al. Different perfusion pattern between acute and chronic pulmonary thromboembolism: Evaluation with two-phase dual-energy perfusion CT. AJR Am J Roentgenol. 2013;200:812–7. doi: 10.2214/AJR.12.8697. [DOI] [PubMed] [Google Scholar]
- 23.Gupta M, Srivastava DN, Seith A, Sharma S, Thulkar S, Gupta R. Clinical impact of multidetector row computed tomography before bronchial artery embolization in patients with hemoptysis: Aprospective study. Can Assoc Radiol J. 2013;64:61–73. doi: 10.1016/j.carj.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Khalil A, Fartoukh M, Parrot A, Bazelly B, Marsault C, Carette MF. Impact of MDCT angiography on the management of patients with hemoptysis. AJR Am J Roentgenol. 2010;195:772–8. doi: 10.2214/AJR.09.4161. [DOI] [PubMed] [Google Scholar]
- 25.Bruzzi JF, Rémy-Jardin M, Delhaye D, Teisseire A, Khalil C, Rémy J. Multi-detector row CT of hemoptysis. Radiographics. 2006;26:3–22. doi: 10.1148/rg.261045726. [DOI] [PubMed] [Google Scholar]
- 26.Larici AR, Franchi P, Occhipinti M, Contegiacomo A, del Ciello A, Calandriello L, et al. Diagnosis and management of hemoptysis. Diagn Interv Radiol. 2014;20:299–309. doi: 10.5152/dir.2014.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cornea AM, McCullough BJ, Mitsumori LM, Gunn ML. Enhancement of the pulmonary arteries and thoracic aorta: Comparison of a biphasic contrast injection and fixed delay protocol with a monophasic injection and a timing bolus protocol. Emerg Radiol. 2015;22:231–7. doi: 10.1007/s10140-014-1269-2. [DOI] [PubMed] [Google Scholar]
- 28.Meena P, Bhalla AS, Goyal A, Sharma R, Kumar A, Srivastva DN, et al. Single-phase split-bolus dual energy computed tomography angiography for evaluation of hemoptysis: A novel application. J Thorac Imaging. 2018;33:366–76. doi: 10.1097/RTI.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 29.Halpern EJ. Triple-rule-out CT angiography for evaluation of acute chest pain and possible acute coronary syndrome. Radiology. 2009;252:332–45. doi: 10.1148/radiol.2522082335. [DOI] [PubMed] [Google Scholar]
- 30.O’Connor JP, Tofts PS, Miles KA, Parkes LM, Thompson G, Jackson A. Dynamic contrast-enhanced imaging techniques: CT and MRI. Br J Radiol. 2011;84:S112–20. doi: 10.1259/bjr/55166688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swensen SJ, Viggiano RW, Midthun DE, Müller NL, Sherrick A, Yamashita K, et al. Lung nodule enhancement at CT: Multicenter study. Radiology. 2000;214:73–80. doi: 10.1148/radiology.214.1.r00ja1473. [DOI] [PubMed] [Google Scholar]
- 32.Qureshi NR, Shah A, Eaton RJ, Miles K, Gilbert FJ, investigators S. Dynamic contrast enhanced CT in nodule characterization: How we review and report. Cancer Imaging. 2016;16:16. doi: 10.1186/s40644-016-0074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Wu N, Cham MD, Song Y. Tumor response in patients with advanced non-small cell lung cancer: Perfusion CT evaluation of chemotherapy and radiation therapy. AJR Am J Roentgenol. 2009;193:1090–6. doi: 10.2214/AJR.08.1367. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Yang ZG, Chen TW, Yu JQ, Sun JY, Chen HJ. First-pass perfusion imaging of solitary pulmonary nodules with 64-detector row CT: Comparison of perfusion parameters of malignant and benign lesions. Br J Radiol. 2010;83:785–90. doi: 10.1259/bjr/58020866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sitartchouk I, Roberts HC, Pereira AM, Bayanati H, Waddell T, Roberts TP. Computed tomography perfusion using first pass methods for lung nodule characterization. Invest Radiol. 2008;43:349–58. doi: 10.1097/RLI.0b013e3181690148. [DOI] [PubMed] [Google Scholar]
- 36.Fraioli F, Anzidei M, Zaccagna F, Mennini ML, Serra G, Gori B, et al. Whole-tumor perfusion CT in patients with advanced lung adenocarcinoma treated with conventional and antiangiogenetic chemotherapy: Initial experience. Radiology. 2011;259:574–82. doi: 10.1148/radiol.11100600. [DOI] [PubMed] [Google Scholar]
- 37.Bhimaniya S, Seith A, Sharma R, Kandasamy D, Kumar A, Mohan A, et al. The 71st Korean Congress of Radiology 2015 September. Seoul, South Korea: Role of dynamic volume perfusion CT in differentiation of histopatholigical subtypes of NSCLC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


