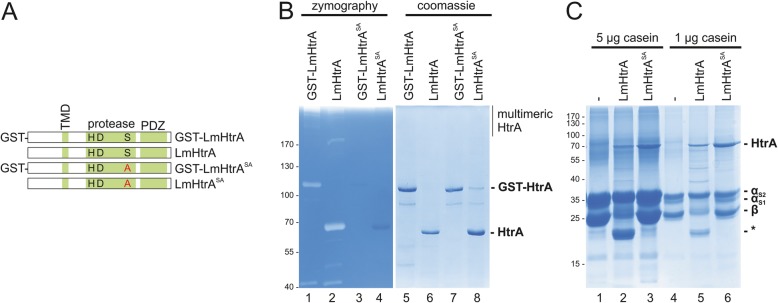
Fig. 2.
Cloning, overexpression and activity of L. monocytogenes HtrA. a HtrA consists of a transmembrane domain, a conserved serine protease domain containing the catalytic triad histidine (H229), aspartic acid (D259), and serine (S343) and a single PDZ domain as annotated by the MEROPS database. Expression constructs for N-terminally, removable GST-tagged HtrA were cloned. Serine 343 in the active center of HtrA was exchanged by an alanine (S343A) to create proteolytic inactive HtrA. b 200 ng of recombinant HtrA proteins were analyzed by casein zymography (left panel) and coomassie-stained protein gels (right panel). c 5 μg or 1 μg casein were incubated with 1 μg LmHtrA wt or LmHtrASA for 16 h at 37 °C. Samples were separated by SDS PAGE and proteins were stained with coomassie blue. Asterisks (*) label casein cleavage products. These assays have been performed as three independent experiments