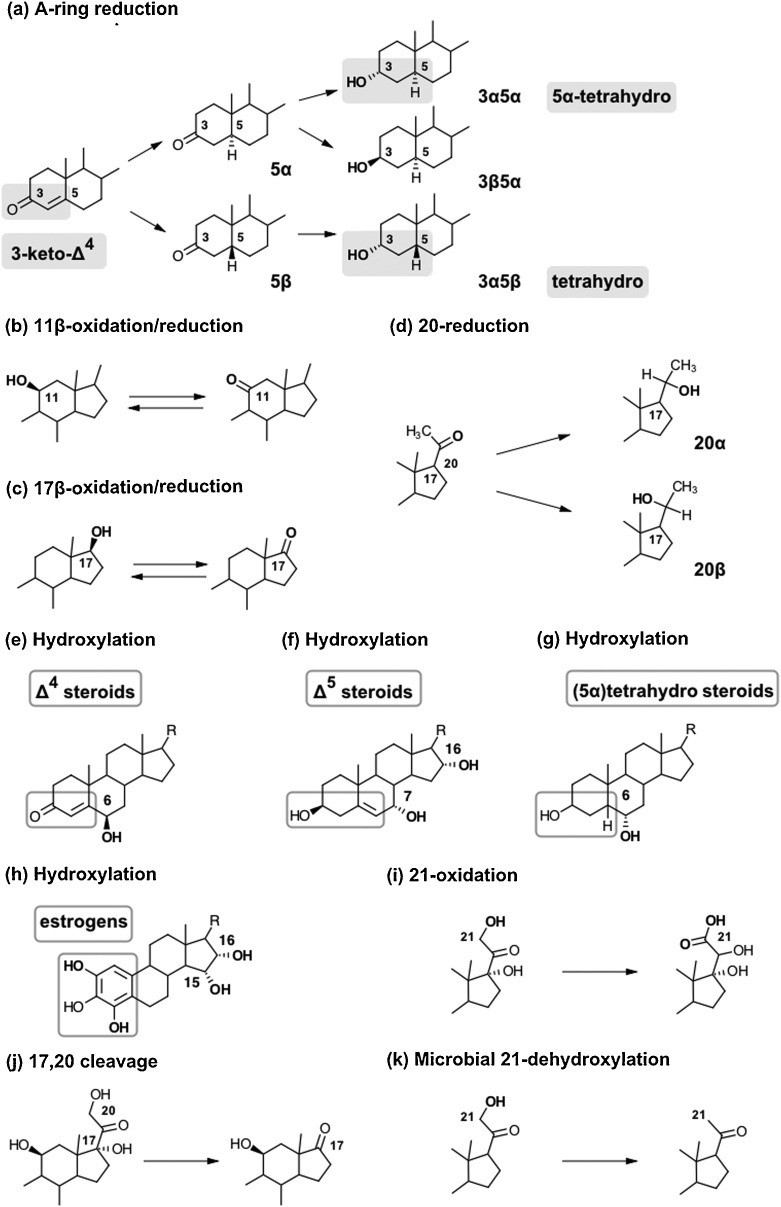
Fig. 4.
Schematic overview of the major phase 1 reactions contributing to steroid metabolism. (a) A-ring reduction to (5α)tetrahydro metabolites. The formation of 3β,5β-tetrahydro metabolites is sterically unfavorable (not shown). (b) 11β-oxidation/reduction by HSD11B1 modulates the bioactivity of glucocorticoids, mineralocorticoids and 11-oxygenated androgens. (c) 17β-oxidation/reduction regulates the bioactivity of androgens and estrogens. (d) 20-reduction to a hydroxy group with α- or β-stereochemistry. (e–h) Hydroxylations: major positions are indicated for different structural steroid classes. (i) 21-oxidation leading to the formation of the so-called cortolic acids from cortisol. (j)17,20-cleavage: cortisol, cortisone and their metabolites can undergo metabolism by 17,20-lyase activity. (k) Microbial 21-dehydroxylation: steroids excreted with bile can undergo metabolism by the gut microbiome prior to reabsorption.