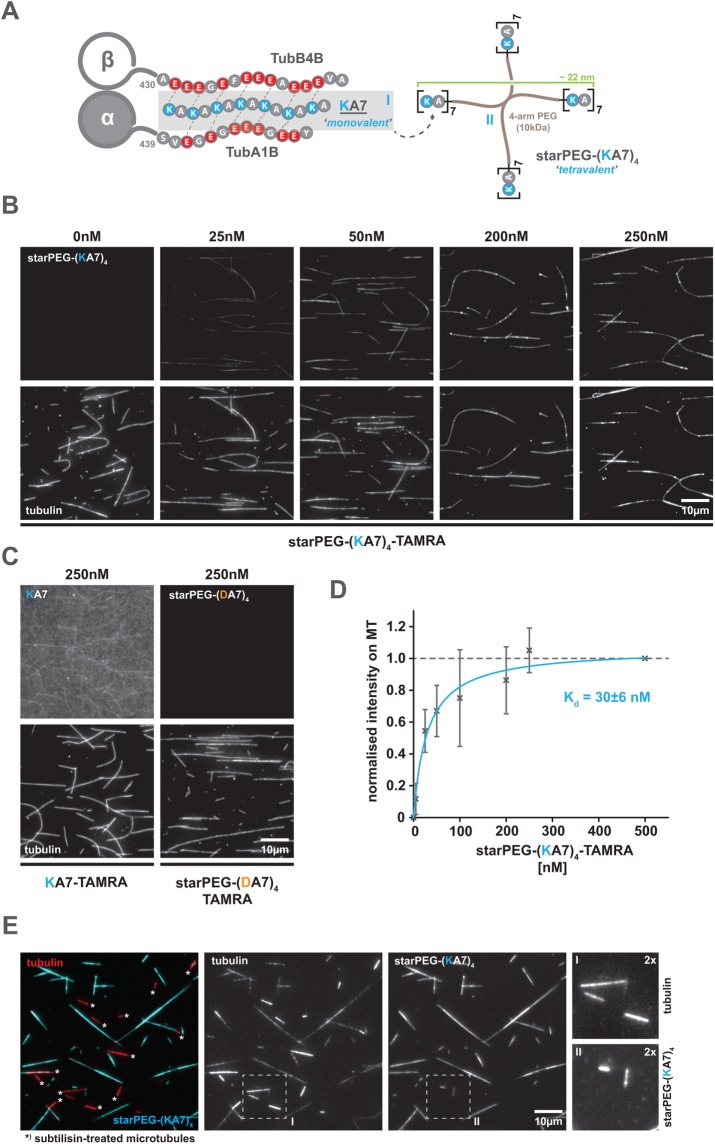
FIGURE 1:
The starPEG-(KA7)4 binds to microtubules at low nanomolar concentrations via electrostatic interactions with the microtubule e-hooks. (A) Schematic overview of the synthetic peptides (KA7, [I] left and starPEG(KA7)4, [II] right) used in this study. Scheme on the left shows the hypothetical interactions of a positively charged KA7 peptide with the negatively charged C-terminal extensions (e-hooks) of a tubulin heterodimer (α-, β-tubulin) exemplified by α-tubulin 1B and β-tubulin 4B. Not drawn to scale. (B) Binding of tetravalent starPEG-(KA7)4-TAMRA molecules (top panel row) to taxol-stabilized Atto647N-labeled microtubules (bottom panel row) at indicated concentrations. Please refer to Supplemental Figure S2A for an extended concentration range. (C) Binding of the charge-inverted starPEG-(DA7)4-TAMRA version (negative control) or a monovalent KA7 peptide to Atto647N-labeled microtubules. Arrangement as in B. Please refer to Supplemental Figure S2B for high-contrast pictures of 0 nM, 5 nM starPEG-(KA7)4-TAMRA, and 250 nM starPEG-(DA7)4-TAMRA. (D) Saturation binding curve of starPEG-(KA7)4-TAMRA showing the concentration-dependent fluorescence intensity of microtubule associated TAMRA normalized to saturating conditions at 500 nM. Error bars depict the SD of five independent experiments. Blue line is a Michaelis-Menten model fit. (E) Binding of starPEG(KA7)4-TAMRA (cyan) to brightly Atto647N-labeled subtilisin-treated (bright red, marked with an asterisk) and dimly Atto647N-labeled nontreated (dim red) CMP-CPP/taxol double-stabilized microtubules. From left to right: overlay, tubulin, starPEG-(KA7)4-TAMRA, twofold magnifications of boxed areas in the single color channels.