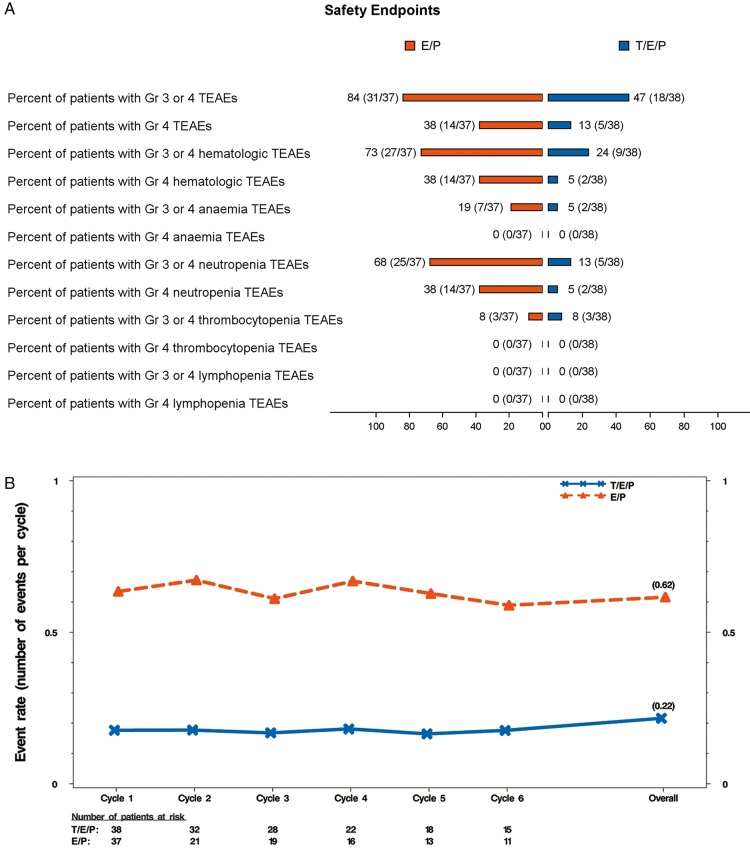
Figure 3.
Safety end points and major adverse hematological events (MAHE) composite. (A) Occurrence of grade 3 or 4 TEAEs (all, hematologic, lineage specific; see supplementary Methods, available at Annals of Oncology online for description) per treatment group represented as percent (number of patients with at least one TEAE/total number of patients per group). (B) Event rate over time for modified MAHE defined using the following events: (1) hospitalization for any reason, (2) febrile neutropenia, (3) death for any reason, (4) dose reduction for any reason, (5) duration of severe (grade 4) neutropenia >5 days, and (6) RBC transfusions occurring on/after 5 weeks after start of treatment. Event rates were calculated using the Poisson method accounting for the ECOG status (0-1 versus 2) as the stratification factor. Events occurring between the first dose and 60 days after the last dose of study drug were included in the event rate over time analysis. orange, E/P + Placebo (E/P); blue, E/P + Trilaciclib 240 mg/m2 (T/E/P). TEAEs, treatment emergent adverse events; MAHE, major adverse hematologic event; RBC, red blood cell.