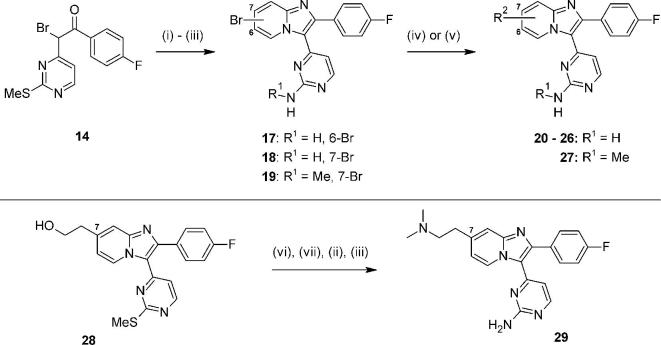
Scheme 3.
Reagents and conditions: (i) 2-amino-4-bromopyridine or 2-amino-5-bromopyridine, EtOH, 4 Å sieves, 80 °C, 18 h, 20–36%; (ii) H2O2, Na2WO4·2H2O, AcOH, MeOH, rt – 50 °C, 18 h; (iii) For R1 = H: NH4OAc, melt, 130 °C, 18 h, 30–52% for two steps; for R1 = Me: Me2NH, THF, 70 °C, 18 h, 56% for two steps; (iv) for R2 = amine: Pd(OAc)2, JohnPhos, R2NH, NaOtBu, dioxane, 100 °C, 18 h, 3–29%; (v) for R2 = alcohol: KOtBu, ROH, NMP, microwave, 170 °C, 10 min, 6%; (vii) MsCl, Et3N, THF, 0 °C, 1 h; (vi) Me2NH, THF, 60 °C, 10 h, 49% for two steps.