Summary:
Understanding variability in cystic fibrosis (CF) health outcomes requires an understanding of factors that goes far beyond CFTR function caused by different gene mutations. Social and environmental factors that influence health have a significant influence on the trajectory of health in CF and in other chronic diseases. In this article, we review demographic factors associated with poorer health outcomes in CF, known and postulated biological mechanisms of these outcomes, and interventions that health care teams can implement that may reduce outcome disparities.
Introduction
There have been tremendous advancements in new therapeutics for cystic fibrosis (CF), including antibiotics, mucolytics, CFTR modulators, over the past decades. This has resulted in improved mortality and morbidity for patients with CF. However, despite these advancements, many patients still suffer significantly from CF and there remains great variability in pulmonary disease progression and severity, even in patients with identical CFTR mutations. There is therefore significant discordance in the CFTR genotype-phenotype relationship. Disease progression and survival are influenced by mode of diagnosis, including meconium ileus and diagnosis by newborn screening, genes other than CFTR, health care provided and health care system factors, and adherence. However, among these and other predictive factors, numerous studies from diverse countries and healthcare settings have shown that that demographic factors, including socioeconomic and ethnic and racial minority status, have a profound influence on health and survival.
In this article, we present demographic factors associated with CF disease variability and poor health outcomes. We review known and postulated biological mechanisms of how these demographic factors influence and interact with the key drivers of health and disease in CF. We conclude with a discussion of the interventions we hypothesize to moderate the effect of demographic risk factors, and that can be implemented by CF care teams to better care for at-risk populations.
Epidemiology
Socioeconomic Status
Low socioeconomic status (SES) has a profound negative effect on health in CF. SES is variously described based on insurance status (public versus private in the US), geographic income measures (median income by zip code in the US), education achieved by a mother (for her child) or by an individual, or composite measures that may include individual income and/or assets. All of these measures are imprecise, but all show significant effects on health. We use definitions interchangeably in this review. People with CF and low SES have increased mortality1–3, worse pulmonary function2,4–6, more frequent pulmonary exacerbations2, and are more likely to have Pseudomonas aeruginosa pulmonary infections4. Despite having more severe disease, patients with low SES are less likely to be accepted for lung transplantation7. Worse nutritional status is seen in patients with lower SES, including lower BMI and shorter height2,4,5,8. The pervasive effects of SES on health seems to occur early in life, without improving or widening over time4.
Low SES negatively affects outcomes in CF in many countries and healthcare systems4,9,10. In the US, patients with Medicaid, a marker of low SES, had a 64% increased risk of hospitalization for CF exacerbation than patients with non-Medicaid insurance11. However, in Canada there was no difference in hospitalization rates between low and high SES patients with CF12. This difference association of SES and hospitalization rates may be due to universal healthcare in Canada, which includes specialty CF centers, CF drug coverage, and funding for travel. Low SES is not associated with fewer clinic visits or less prescription of acute or chronic medication in the U.S.13,14. However, a UK study showed therapeutic variation by SES after adjusting for disease severity with the lowest SES receiving more intravenous antibiotics and nutritional treatments, but fewer prescriptions for inhaled antibiotics or DNase, despite the UK having a national healthcare system with no out of pocket costs for medication4. Medication adherence is decreased in low SES in diseases other than CF15. Medication adherence by SES has not been studied in CF, however there was decreased adherence to airway clearance therapies in patients with low SES6. The financial cost of the high medication burden may influence adherence more in low SES than high SES and should be investigated.
While low SES is a key driver of poor health outcomes in the CF population, individuals are also affected by family and neighborhood environment. Being in poverty in a poor county with few resources for families, low-quality schools, and limited pathways for economic gains has more of a negative impact on health trajectory for children with health conditions or disabilities than being in poverty in a wealthy county16. Community support and personal liquid assets or savings have a benefit on the impact of poverty on a child’s health, especially with unexpected medical costs, but can be difficult to measure16. Incorporating healthcare costs, including the extent to which health insurance assists with affording those costs, is difficult but increasingly relevant in understanding SES and CF.
Patients with CF and their families are at risk for a decline in SES. Families with CF are at greater risk of poverty due to both increased expenditures, including costs from medical care, travel to appointments, and food expenditures to meet the caloric needs in CF. Decreased income is also common as caregivers frequently decrease their hours working to provide care. Annual medical costs for CF are high and escalate as disease severity worsens17. Housing insecurity is also a risk, as the majority of families with special needs have been late in housing payments, and 1 in 5 were faced with foreclosure18.
Race and Ethnicity
The US population is becoming more racially and ethnically diverse, and children are more diverse than the general population. While CF is most prevalent in non-Hispanic whites, an increasing proportion of patients are minorities, mirroring general population demographics19. Race and ethnicity are social constructs, but have important health implications. The terms “African American” and “Black” are both used in the literature, as are “Hispanic”, “Latino/a” or the gender-neutral “Latinx”. For consistency we have utilized the terms “Black” and “Hispanic”. The term “minority” is used for race and ethnicity other than non-Hispanic white. The studies discussed primarily utilize self-reported race and ethnicity.
Despite great advancements in CF care and outcomes, minorities with CF suffer worse health outcomes, even after adjustment for SES. Hispanic patients have a higher rate of mortality than do non-Hispanic white patients20,21. Black patients have more severe pulmonary imaging findings and more respiratory symptoms at diagnosis than white patients22. Hispanic and Black patients with CF have worse pulmonary function than non-Hispanic white patients23,24. In the Hispanic population, the gap in pulmonary function is present at 6 years of age, but remains stable, indicating more severe lung disease early in life23. The ethnic disparity in CF morbidity and mortality is not uniform across the United States, but varies significantly by region25.
Worse outcomes in Hispanic patients occur in spite of higher body mass index (BMI), higher likelihood of pancreatic sufficiency, and presence of milder CFTR mutations23,26, all otherwise associated with better CF health outcomes. This paradox is particularly striking given that in the general population, US persons of Hispanic ethnicity have a longer life expectancy than non-Hispanic whites. The increased morbidity and mortality in Hispanic patients must be driven by something other than nutritional status or CFTR genetics. There are many unmeasured and unstudied factors that could contribute to the observed disparities that specifically affect Hispanics, such as language spoken, health literacy, medication adherence, and acculturation level.
Advances in CF have included early diagnosis, new medications, and other therapies; however, these advances may not always benefit minority patients. Minorities are less likely to be detected on prenatal and newborn screening tests due to different frequencies of CFTR mutations and use of DNA panels representing common mutations found in non-Hispanic white patients27. This can lead to delayed diagnosis in minorities. Minorities are under-represented in pharmaceutical clinical trials of non-modulator therapies in proportion to their representation in the CF population28. Some commonly used CF medications, including dornase alfa, were not studied in any minorities. Extrapolating results to minorities can be dangerous; many medications have significant racial and ethnic differences in therapeutic responses, drug metabolism, and adverse effects29.
Minorities with CF are likely to experience racism that may contribute to negative health outcomes in the general population. A recent metanalysis reviewing 333 studies in the general population on reported racism and health outcomes demonstrated significant associations between racism and mental and general health outcomes30. Age, sex, birthplace and education level did not reduce these health effects. Ethnicity moderated the effect of racism on negative mental and physical health, with a stronger association between racism and negative mental health in Asian and Hispanic participants compared to Black participants. Furthermore, the association between racism and physical health was stronger for Hispanic participants than for Black participants.
There is evidence for differences in health care delivery to racial and ethnic minorities that may occur due to implicit associations. Implicit association describes thoughts and feelings that exist outside of conscious awareness and are therefore difficult to consciously acknowledge and control. A 2015 systematic review of implicit racial/ethnic biases among health care professionals found low to moderate levels of implicit racial/ethnic bias were found among health care professionals31. Implicit bias was significantly related to patient–provider interactions, treatment decisions, treatment adherence, and patient health outcomes31. Implicit biases were more often significantly related to patient–provider interactions and health outcomes than treatment processes31. A systematic review in 2018 of implicit bias in health care providers found evidence of pro-White or light-skin and anti-Black, Hispanic, American Indian or dark-skin bias among health care providers32. In the 14 studies that examined implicit bias and healthcare outcomes using clinical situations, 8 found no association between implicit bias and patient care while 6 studies found an association between higher implicit bias and disparities in treatment recommendations, expectations of therapeutic bonds, pain management, and empathy32. The extent of implicit bias in CF has not been investigated but should be studied in each CF care center. Implicit biases of CF care providers may impact the care provided.
Sex
Mortality is higher in girls and women with CF compared to boys and men, a finding not easily explained by differences in pulmonary function, infections, or nutrition33–35. Female sex is associated with more pulmonary exacerbations and with earlier age at first exacerbation36. Female sex is also associated with more variable pulmonary function37. Not surprisingly, pulmonary function decline in females with low physical activity is steeper than males, while females that are physically active have less decline38. Pseudomonas aeruginosa, Staph aureus (MSSA, MRSA), H. influenzae, A. xylosoxidans, B. cepacia, Aspergillus species, and nontuberculous mycobacteria are acquired at an earlier age in female patients, which may contribute to higher morbidity and mortality39–41. Female sex is associated with increased risk of CF-related diabetes (CFRD), diagnosis due to symptoms at a later age, and have increased mortality compared to males42–44.
Despite having worse outcomes, females are diagnosed at a later age than males, especially when presenting with respiratory symptoms45. Historically, this may be due to unconscious gender bias in referring girls for sweat chloride testing. Widespread newborn screening could decrease this bias. However, diagnostic delay persisted in Wisconsin even after newborn screening began45.
Adherence to therapies is lower in girls and women with CF, who are more likely to skip medications or chest physiotherapy, decrease caloric intake to obtain thinner body stature, and suppress cough46. Female patients may be thinner due to body image issues and exacerbated by praise for an asthenic appearance. In one study, female patients who were underweight were more likely than male patients to consider their weight to be normal47.
CF seems to have more of an impact in the lives of girls and women compared to boys and men. Multiple studies have found that female CF patients have lower health-related quality of life compared to males, even with adjustment for disease severity48,49. Female patients also have more emotional impact from their CF than their male counterparts, with higher rates of emotional strain, worry about the future, lower self-esteem, and greater discouragement46.
Biology
Diet and Nutrition
Nutritional status is highly correlated with pulmonary function and survival in CF. Poor nutritional status is associated with more severe pulmonary disease that increases caloric demands leading to worse nutritional status. Furthermore, many CF co-morbidities, including CFRD, pancreatic insufficiency, osteoporosis, intestinal resection for meconium ileus, distal intestinal obstruction syndrome, pancreatitis, short stature, and eating disorders, require special nutritional attention. The CF population is at increased risk of disordered eating which can further impact nutritional status50.
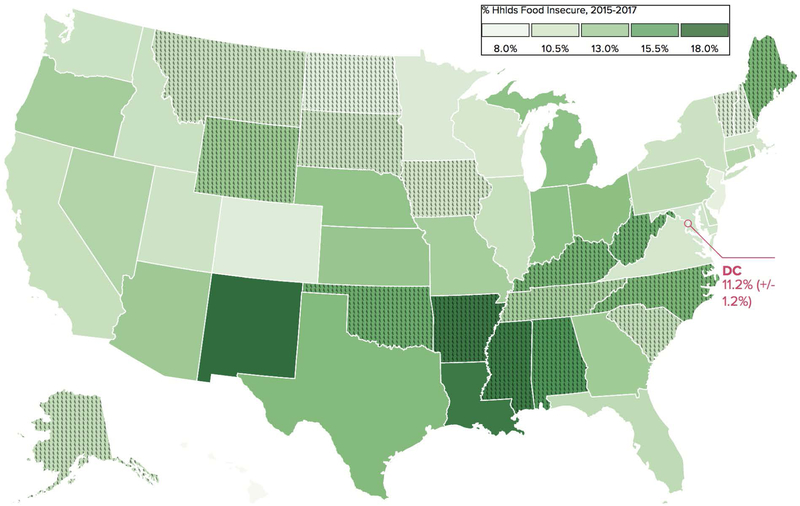
Food insecurity, the limited or uncertain access to food, impacts 15 million US households51 (Figure 1). Households with a child with any special needs are 24% more likely to have food insecurity compared to a family without special needs52. Children with special needs who are poor are 50% more likely to have food insecurity52. This is especially concerning in CF, as the high-calorie, high-fat, and high-protein diet required is costly. Within the energy dense CF diet, it is important to have nutrient dense foods, which are more expensive, rather than nutrient poor foods which are typically high in sugar and saturated fat53. Food insecurity is more prevalent in families with CF despite these families having a high median income, with approximately one quarter of families reporting food insecurity54. Families that are Black, Hispanic, immigrants, or located outside of a metropolitan area are at higher risk for food insecurity55. In many states, the percentage of families enrolled in federal food assistance programs is less than the percentage reporting food insecurity54. Furthermore, these assistance programs, may not provide enough benefits to cover the increased nutritional needs of CF.
Figure 1:
Household Food Insecurity Rates By State, 2015–2017
Citation: Food Research & Action Center
Environmental Exposures
In the general population, early life exposure to air pollution and pesticides is associated with abnormal pulmonary function and respiratory tract disease. In CF, air pollution exposure is associated with pulmonary exacerbation and with Pseudomonas aeruginosa acquisition56,57. Minority children are more likely to be exposed to air pollution than non-Hispanic white children, especially if they live in a low-income neighborhood58. There is a heterogeneous response to air pollution with Hispanic children more likely to require hospitalization for asthma from fine particulate matter exposure than non-Hispanic white children59. In California, areas with highest exposure of pesticides had the highest percentage of Hispanic residents60. Hispanic children living in California are disproportionately exposed to pesticides as their homes are more likely to be situated closer to the fields where pesticides are used60. These environmental exposures may place disproportionate health risks on Hispanic children who are already vulnerable based on socioeconomic and other disparities.
Environmental tobacco smoke exposure is associated with adverse outcomes in CF, as it is in people with other pulmonary diseases and those without lung disease5,61,62. While cigarette smoking is lower in CF population than the general population, secondhand tobacco exposure in CF patients is similar to the general population19,63. In addition to inflammatory effects in the respiratory tract, tobacco exposure inhibits CFTR function64. Heavy tobacco smoke exposure is associated with lower pulmonary function61,62, increased hospitalizations61, lower weight43 in CF. Children with low SES are disproportionately exposed to tobacco smoke5,62.
E-cigarette use has greatly increased due to the false assumption they are safe. E-cigarettes are now the most commonly used form of tobacco in teenagers65. Patients or families using e-cigarettes are more likely to progress to using cigarettes. Some of the e-cigarette vapor contains high levels of nickel, chromium, and cadmium which are carcinogens that can negatively affect pulmonary function66.
Adverse childhood experiences
While the prevalence of adverse childhood experiences (ACEs), traumatic events occurring before age 18 years old, in CF is unknown, ACEs are likely to contribute to worse outcomes in people with CF. ACEs span a wide range of experiences that typically encompass abuse, neglect, and household factors. Most ACEs studies capture these categories: emotional, physical, or sexual abuse; exposure to violence, neglect, or discrimination; parental mental illness, substance abuse, divorce or death, resulting in toxic stress. The first study of ACEs evinced a dose dependent relationship between the number of maltreatments in childhood and poor social and health outcomes in adults67.
Toxic stress in childhood leads to dysfunction of the neuroendocrine-immune network. Diminished cortisol can allow persistent inflammation, while excess cortisol can suppress immune response68. Dysregulation of the immune and endocrine systems, particularly during periods of development, leaves children vulnerable for a wide array of health problems69. ACEs are associated with numerous adult health issues including depression, alcoholism, cancer, and heart disease, and also increase the number of health problems per individual67,70.
As seen in Figure 2, 46% of children have at least one ACE. ACEs have been linked to learning and behavior problems, obesity, eating disorders, depressed mood, posttraumatic stress disorder symptoms, and suicides in youth71–75. ACEs are more prevalent in children of lower socioeconomic status, more complex health needs, and racial and ethnic minorities (Figure 2). The impact of ACEs on childhood chronic disease is less well understood. However, recent studies have found a correlation between the number of ACEs and the development of pediatric asthma and the number of unmet care needs in children with autism spectrum disorder76,77. ACEs have not been investigated in CF, but may play an important role in disease variability.
Figure 2.
2016–2017 National Prevalence of Adverse Childhood Experiences
A. Prevalence of ACEs in the US is the overall population and in children with more complex health care needs. B. ACE stratified by household income. FPL represents the Federal Poverty Line. C. ACEs by race/ethnicity. Error Bars represents 95% confidence intervals. *Percentages do not sum to 100 due to rounding. From the National Survey of Children’s Health. Used with permission.
Child and Adolescent Health Measurement Initiative. 2016–2017 National Survey of Children’s Health (NSCH) data query. Data Resource Center for Child and Adolescent Health supported by Cooperative Agreement U59MC27866 from the U.S. Department of Health and Human Services, Health Resources and Services Administration’s Material and Child Health Bureau (HRSA MCHB). Retrieved [05/16/19] from www.childhealthdata.org. CAHMI: www.cahmi.org.
The epigenetics of poverty
The biologic mechanisms related to poverty are starting to be elucidated. A recent report evaluated levels of DNA methylation at CpG sites across the genome and evaluated the association with SES in a cohort of young adults in the Philippines78. In comparison with high SES, low SES was associated with increased methylation at 1,777 sites, and decreased methylation at 769 sites, after adjustment for multiple comparison, represented in Figure 3. Over-representation of biological pathways and genes related to immune function and inflammation were noted in the study. Higher levels of inflammation or altered immune function may explain the observed lower pulmonary function, increased pulmonary exacerbations, and higher rates of Pseudomonas aeruginosa infections in CF with lower SES and should be investigated.
Figure 3:
Venn diagram showing areas of DNA methylation significantly associated with SES and its components (household assets, education, household income), based on the contrast between low/low and high/high groups within each SES component.
Interventions
Alongside the many therapeutic advances being made in CF to reduce morbidity and mortality, the discussed demographic disparities also need to be addressed to ensure that health outcomes continue to improve equitably for all patients with CF. While systematic societal efforts are necessary to reduce or eliminate poverty and its myriad effects on human health, we propose interventions that can be implemented by healthcare providers and/or within healthcare systems (Figure 4). These interventions have not been studied systematically in CF, and some have limited evidence for interventions in other populations. Nevertheless, the impact of demographic factors is so substantial that considering specific actions is necessary.
Figure 4.
Four approaches to address health disparities in clinical practice.
Ways to Reduce Health Care System Bias
Addressing Implicit Bias
The Implicit Association Test (IAT) measures implicit bias via tests of automatic associations between concepts. There are few studies examining the effects of IAT for health care providers or programs to reduce implicit bias32. Nevertheless, widespread IAT as a self-assessment tool can raise awareness to the unconscious biases that may affect communication and patient care. Project Implicit (www.implicit.harvard.edu) provides free access to a variety of these tests and has a number of services for organizations interested in reducing the negative impact of implicit bias.
Language, Culture, And Healthcare Team Diversity
Appropriate medical interpretation is essential for provision of even adequate health care to patients and their families who do not speak the language of the health care professionals caring for them. Cultural competency training is proposed to improve patient outcomes. A 2018 systematic review of 16 studies of health care professionals demonstrated that diverse programs had positive effects on practitioner knowledge and attitudes, but few studies evaluated interventions79. Increasing diversity among the health care professions is an important strategy to reduce the effects of bias and is the focus of efforts by the United States Department of Health and Human Services80.
Food Insecurity Screening
Since food insecurity is prevalent in CF, the Cystic Fibrosis Foundation has formed a task force to address this important issue at CF centers. CF centers should identify and assist patients with food insecurity. Algorithms for routine screening can be added into the registration/intake process of visits and appointments. Screening can be done verbally or written in the patient’s preferred language. There is a simple 2-question true or false screen for food insecurity “The Hunger Vital Sign” that has been developed by the American Academy of Pediatrics: “I worried about not having enough to eat. I tried to not eat a lot so our food would last”81. Electronic health records (EHR) have been used successfully to screen patients and share referral information about government and community programs for food resources, such as Supplemental Nutrition Assistance Program (SNAP), Summer Food Assistance Programs (SFAP), and local food banks. The Hunger Vital Sign is already built into Epic’s Foundation System. Positive screens can be tracked with the ICD-10-CM Diagnosis Code Z59.4 (lack of adequate food and safe drinking water). Particular sensitivity and attention should be given prior to referring immigrant patients to federal nutrition programs, especially when families have mixed citizenship status.
Food insecurity is never an isolated problem. Targeting other hardships such as housing or energy costs can allow for enough funds for food82. Educating families about programs to spread their energy costs evenly over the year can be beneficial. There are lower levels of food insecurity after receiving housing assistance. There are many unreimbursed medical costs with CF, such as insurance copayments, deductibles, and vitamin costs. Medication and treatment adherence is improved with addressing food insecurity, especially with more costly therapies83. One third of families with food insecurity had to choose between paying for medical care or purchasing food84. CF care providers should educate families and advocate for insurance that covers more of the medical costs to shield families from health shocks, such as prolonged hospitalizations, which can cause a family to fall into food insecurity and poverty.
Air Pollution and Tobacco Exposure
Avoidance of indoor and outdoor air pollution is beneficial and possible for patients with CF. Patients in high risk areas, such as areas near wildfires, should be counseled about using protective masks that filter airborne particulate matter to reduce the effect of air pollution. Proper fit and usage of masks is essential for protective effects. Masks should be kept on hand for use in cases of emergent air pollution as there are often shortages at times of need. Monitoring air quality from the Environmental Protective Agency (EPA) or Purple Air is useful to determine current air pollution risk levels. At times of heavy air pollution, patients should be counseled to stay indoors with windows kept shut. Portable air filtration systems are affordable and can reduce the level of indoor air pollution. While avoiding living and working in high air pollution areas, such as those caused by automobile traffic, is ideal, it is often not possible. Impoverished families and minorities who are most likely to live in high air pollution areas should be targeted for counseling58.
Patients and families should be screened for tobacco use and exposure at routine visits and hospitalizations. Resources on tobacco cessation should be given to any patient or family with tobacco exposure. If smoking cessation is not possible, exposure should be reduced by implementing family “no smoking” rules indoors and in vehicles. CF providers should pay attention to screening for e-cigarette use, now the most commonly used form of tobacco in teenagers85.
Mitigating Adverse Childhood Experiences (ACEs)
The American Academy of Pediatrics recognizes the toxic and compounding nature of ACEs and has announced a need for pediatricians to bolster screening and pursue childhood interventions68,69. More research is required to elucidate the most effective intercessions to ameliorate the short- and long-term impacts from childhood maltreatment86. New efforts to counsel children with trauma-informed care techniques and to educate parents demonstrates potential areas for integrated care86–88. Focusing on techniques that bolster resiliency and mindfulness have been associated with countering some of the negative impacts of ACEs, including higher rates of school engagement among children with multiple ACEs87,88. There are currently no studies that have documented the prevalence of ACEs in children with CF. Counseling and techniques that emphasize resiliency and mindfulness coupled with enhanced screening for ACEs in the CF setting may help mitigate the burden of ACEs87,88. In the US, accredited CF Centers have social workers and often psychologists, making intervention and referral highly feasible in practice.
Inclusion of minorities in clinical research
Exclusion of women and minority groups has been an issue for decades in both observational and interventional research. To address this, US federal law now requires that “In conducting or supporting clinical research … the Director of NIH shall … ensure that (a) women are included as subjects in each project of such research; and (b) members of minority groups are included in such research 492B(a)(1)”89. It is also specifically forbidden to exclude women and minorities based on increased cost that may result from inclusion. While these legal requirements are applicable only to NIH-funded research, research sponsored by non-profit and industry sources should strive to be equally inclusive. In CF programs conducting clinical trials, making special efforts to include patients who have fewer resources or are minorities can be implemented during pre-screening activities. These inclusion efforts might include anticipating need for providing transportation (rather than reimbursing a family with a reliable means of transportation), the need for translation of study materials and consent forms, and the need to have interpreters for study visits.
Conclusions
Epidemiologic reports demonstrate incontrovertible evidence of demographic disparities in CF health outcomes. The biologic mechanisms that are associated with low SES status are beginning to be elucidated. While there is little CF-specific literature, interventions that may reduce disparities can be implemented by health care teams at CF Centers, and studying their impact is important. While proposed interventions can be implemented by health care professionals, the authors recognize the importance of public policy, including educational, environmental, health care and other strategies to reduce disparities in the entire population.
Acknowledgments
Financial disclosures: MEM is supported by the NHLBI (1K23HL133437–01A1) and Cystic Fibrosis Foundation Therapeutics (MCGARR16A0). SAM is supported by the Northwestern University Clinical and Translational Sciences Institute through the National Center for Advancing Translational Sciences (UL1TR001422).
Footnotes
No conflicts of interest for MEM, WAW, SAM
References
- 1.Barr HL, Britton J, Smyth AR, Fogarty AW. Association between socioeconomic status, sex, and age at death from cystic fibrosis in England and Wales (1959 to 2008): cross sectional study. Bmj. 2011;343:d4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schechter MS, Margolis PA. Relationship between socioeconomic status and disease severity in cystic fibrosis. The Journal of pediatrics. 1998;132(2):260–264. [DOI] [PubMed] [Google Scholar]
- 3.O’Connor GT, Quinton HB, Kneeland T, Kahn R, Lever T, Maddock J, Robichaud P, Detzer M, Swartz DR. Median household income and mortality rate in cystic fibrosis. Pediatrics. 2003;111(4):e333–e339. [DOI] [PubMed] [Google Scholar]
- 4.Taylor-Robinson DC, Smyth RL, Diggle PJ, Whitehead M. The effect of social deprivation on clinical outcomes and the use of treatments in the UK cystic fibrosis population: a longitudinal study. The Lancet Respiratory Medicine. 2013;1(2):121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ong T, Schechter M, Yang J, Peng L, Emerson J, Gibson RL, Morgan W, Rosenfeld M, Group ES. Socioeconomic status, smoke exposure, and health outcomes in young children with cystic fibrosis. Pediatrics. 2017;139(2):e20162730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oates GR, Stepanikova I, Gamble S, Gutierrez HH, Harris WT. Adherence to airway clearance therapy in pediatric cystic fibrosis: socioeconomic factors and respiratory outcomes. Pediatric pulmonology. 2015;50(12):1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quon BS, Psoter K, Mayer-Hamblett N, Aitken ML, Li CI, Goss CH. Disparities in access to lung transplantation for patients with cystic fibrosis by socioeconomic status. American journal of respiratory and critical care medicine. 2012;186(10):1008–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balmer DF, Schall JI, Stallings VA. Social disadvantage predicts growth outcomes in preadolescent children with cystic fibrosis. Journal of Cystic Fibrosis. 2008;7(6):543–550. [DOI] [PubMed] [Google Scholar]
- 9.Sánchez-Domínguez CN, Reyes-López MÁ, Bustamante A, Trevino V, Martínez-Rodríguez HG, Rojas-Martínez A, Barrera-Saldaña HA, Ortiz-López R. Low-income status is an important risk factor in North East Mexican patients with cystic fibrosis. Revista de investigacion clinica; organo del Hospital de Enfermedades de la Nutricion. 2014;66(2):129–135. [PubMed] [Google Scholar]
- 10.Taylor-Robinson DC, Thielen K, Pressler T, Olesen HV, Diderichsen F, Diggle PJ, Smyth R, Whitehead M. Low socioeconomic status is associated with worse lung function in the Danish cystic fibrosis population. European Respiratory Journal. 2014;44(5):1363–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schechter MS, Shelton BJ, Margolis PA, Fitzsimmons SC. The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. American journal of respiratory and critical care medicine. 2001;163(6):1331–1337. [DOI] [PubMed] [Google Scholar]
- 12.Stephenson A, Hux J, Tullis E, Austin PC, Corey M, Ray J. Socioeconomic status and risk of hospitalization among individuals with cystic fibrosis in Ontario, Canada. Pediatric pulmonology. 2011;46(4):376–384. [DOI] [PubMed] [Google Scholar]
- 13.Schechter MS, McColley SA, Silva S, Haselkorn T, Konstan MW, Wagener JS, Investigators, Fibrosis CotESoC. Association of socioeconomic status with the use of chronic therapies and healthcare utilization in children with cystic fibrosis. The Journal of pediatrics. 2009;155(5):634–639. e634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schechter MS, McColley SA, Regelmann W, Millar SJ, Pasta DJ, Wagener JS, Konstan MW, Morgan WJ, Investigators, Fibrosis CotESoC. Socioeconomic status and the likelihood of antibiotic treatment for signs and symptoms of pulmonary exacerbation in children with cystic fibrosis. The Journal of pediatrics. 2011;159(5):819–824. e811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apter AJ, Reisine ST, Affleck G, Barrows E, ZuWallack RL. Adherence with twice-daily dosing of inhaled steroids: socioeconomic and health-belief differences. American journal of respiratory and critical care medicine. 1998;157(6):1810–1817. [DOI] [PubMed] [Google Scholar]
- 16.National Academies of Sciences E, Medicine. A Roadmap to Reducing Child Poverty. Washington, DC: The National Academies Press; 2019. [PubMed] [Google Scholar]
- 17.Van Gool K, Norman R, Delatycki MB, Hall J, Massie J. Understanding the costs of care for cystic fibrosis: an analysis by age and health state. Value in health. 2013;16(2):345–355. [DOI] [PubMed] [Google Scholar]
- 18.DeJong NA, Wood CT, Morreale MC, Ellis C, Davis D, Fernandez J, Steiner MJ. Identifying social determinants of health and legal needs for children with special health care needs. Clinical pediatrics. 2016;55(3):272–277. [DOI] [PubMed] [Google Scholar]
- 19.Cystic Fibrosis Foundation Patient Registry, 2016. Annual Data Report Bethesda, Maryland. [Google Scholar]
- 20.Buu MC, Sanders LM, Mayo JA, Milla CE, Wise PH. Assessing differences in mortality rates and risk factors between Hispanic and non-Hispanic patients with cystic fibrosis in California. CHEST Journal. 2016;149(2):380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rho J, Ahn C, Gao A, Sawicki GS, Keller A, Jain R. Disparities in Mortality of Hispanic Cystic Fibrosis Patients in the United States: A National and Regional Cohort Study. American journal of respiratory and critical care medicine. 2018(ja). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McColley SA, Rosenstein BJ, Cutting GR. Differences in expression of cystic fibrosis in blacks and whites. American journal of diseases of children. 1991;145(1):94–97. [DOI] [PubMed] [Google Scholar]
- 23.McGarry ME, Neuhaus J, Nielson DW, Burchard EG, Ly NP. Pulmonary function disparities exist and persist in Hispanic patients with cystic fibrosis: A longitudinal analysis. Pediatric pulmonology. 2017;52(12):1550–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamosh A, FitzSimmons SC, Macek M Jr, Knowles MR, Rosenstein BJ, Cutting GR. Comparison of the clinical manifestations of cystic fibrosis in black and white patients. The Journal of pediatrics. 1998;132(2):255–259. [DOI] [PubMed] [Google Scholar]
- 25.McGarry ME, Neuhaus JM, Nielson DW, Ly NP. Regional variations in longitudinal pulmonary function: A comparison of Hispanic and non‐Hispanic subjects with cystic fibrosis in the United States. Pediatric pulmonology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watts KD, Seshadri R, Sullivan C, McColley SA. Increased prevalence of risk factors for morbidity and mortality in the US Hispanic CF population. Pediatric pulmonology. 2009;44(6):594–601. [DOI] [PubMed] [Google Scholar]
- 27.Watts KD, Layne B, Harris A, McColley SA. Hispanic infants with cystic fibrosis show low CFTR mutation detection rates in the Illinois newborn screening program. Journal of genetic counseling. 2012;21(5):671–675. [DOI] [PubMed] [Google Scholar]
- 28.McGarry ME, McColley SA. Minorities are underrepresented in clinical trials of pharmaceutical agents for cystic fibrosis. Annals of the American Thoracic Society. 2016;13(10):1721–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carson P, Ziesche S, Johnson G, Cohn JN, Group V-HFTS. Racial differences in response to therapy for heart failure: analysis of the vasodilator-heart failure trials. Journal of cardiac failure. 1999;5(3):178–187. [DOI] [PubMed] [Google Scholar]
- 30.Paradies Y, Ben J, Denson N, Elias A, Priest N, Pieterse A, Gupta A, Kelaher M, Gee G. Racism as a determinant of health: a systematic review and meta-analysis. PloS one. 2015;10(9):e0138511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall WJ, Chapman MV, Lee KM, Merino YM, Thomas TW, Payne BK, Eng E, Day SH, Coyne-Beasley T. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. American journal of public health. 2015;105(12):e60–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maina IW, Belton TD, Ginzberg S, Singh A, Johnson TJ. A decade of studying implicit racial/ethnic bias in healthcare providers using the implicit association test. Social Science & Medicine. 2018;199:219–229. [DOI] [PubMed] [Google Scholar]
- 33.Rosenfeld M, Davis R, FitzSimmons S, Pepe M, Ramsey B. Gender gap in cystic fibrosis mortality. American journal of epidemiology. 1997;145(9):794–803. [DOI] [PubMed] [Google Scholar]
- 34.Kulich M, Rosenfeld M, Goss CH, Wilmott R. Improved survival among young patients with cystic fibrosis. The Journal of pediatrics. 2003;142(6):631–636. [DOI] [PubMed] [Google Scholar]
- 35.Nick JA, Chacon CS, Brayshaw SJ, Jones MC, Barboa CM, Clair CGS, Young RL, Nichols DP, Janssen JS, Huitt GA. Effects of gender and age at diagnosis on disease progression in long-term survivors of cystic fibrosis. American journal of respiratory and critical care medicine. 2010;182(5):614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Block JK, Vandemheen KL, Tullis E, Fergusson D, Doucette S, Haase D, Berthiaume Y, Brown N, Wilcox P, Bye P. Predictors of pulmonary exacerbations in patients with cystic fibrosis infected with multi-resistant bacteria. Thorax. 2006;61(11):969–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patterson JM, Wall M, Berge J, Milla C. Associations of psychosocial factors with health outcomes among youth with cystic fibrosis. Pediatric pulmonology. 2009;44(1):46–53. [DOI] [PubMed] [Google Scholar]
- 38.Schneiderman-Walker J, Wilkes D, Strug L, Lands L, Pollock S, Selvadurai H, Hay J, Coates A, Corey M. Sex differences in habitual physical activity and lung function decline in children with cystic fibrosis. The Journal of pediatrics. 2005;147(3):321–326. [DOI] [PubMed] [Google Scholar]
- 39.Demko CA, Byard PJ, Davis PB. Gender differences in cystic fibrosis: Pseudomonas aeruginosa infection. Journal of clinical epidemiology. 1995;48(8):1041–1049. [DOI] [PubMed] [Google Scholar]
- 40.Maselli JH, Sontag MK, Norris JM, MacKenzie T, Wagener JS, Accurso FJ. Risk factors for initial acquisition of Pseudomonas aeruginosa in children with cystic fibrosis identified by newborn screening. Pediatric pulmonology. 2003;35(4):257–262. [DOI] [PubMed] [Google Scholar]
- 41.Harness-Brumley CL, Elliott AC, Rosenbluth DB, Raghavan D, Jain R. Gender differences in outcomes of patients with cystic fibrosis. Journal of women’s health. 2014;23(12):1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milla CE, Billings J, Moran A. Diabetes is associated with dramatically decreased survival in female but not male subjects with cystic fibrosis. Diabetes Care. 2005;28(9):2141–2144. [DOI] [PubMed] [Google Scholar]
- 43.Van den Berg J, Kouwenberg J, Heijerman H. Demographics of glucose metabolism in cystic fibrosis. Journal of Cystic Fibrosis. 2009;8(4):276–279. [DOI] [PubMed] [Google Scholar]
- 44.Marshall B, Butler S, Stoddard M, Moran A, Liou T, Morgan WJ. Epidemiology of cystic fibrosis-related diabetes. The Journal of pediatrics. 2005;146(5):681–687. [DOI] [PubMed] [Google Scholar]
- 45.Lai H-C, Kosorok MR, Laxova A, Makholm LM, Farrell PM. Delayed diagnosis of US females with cystic fibrosis. American journal of epidemiology. 2002;156(2):165–173. [DOI] [PubMed] [Google Scholar]
- 46.Patterson JM, Wall M, Berge J, Milla C. Gender differences in treatment adherence among youth with cystic fibrosis: development of a new questionnaire. Journal of Cystic Fibrosis. 2008;7(2):154–164. [DOI] [PubMed] [Google Scholar]
- 47.Walters S Sex differences in weight perception and nutritional behaviour in adults with cystic fibrosis. Journal of Human Nutrition and Dietetics. 2001;14(2):83–91. [DOI] [PubMed] [Google Scholar]
- 48.Arrington-Sanders R, Michael SY, Tsevat J, Wilmott RW, Mrus JM, Britto MT. Gender differences in health-related quality of life of adolescents with cystic fibrosis. Health and Quality of Life Outcomes. 2006;4(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uchmanowicz I, Jankowska-Polańska B, Wleklik M, Rosinczuk-Tonderys J, Dębska G. Health-related quality of life of patients with cystic fibrosis assessed by the SF-36 questionnaire. Advances in Respiratory Medicine. 2014;82(1):10–17. [DOI] [PubMed] [Google Scholar]
- 50.Conviser JH, Fisher SD, McColley SA. Are children with chronic illnesses requiring dietary therapy at risk for disordered eating or eating disorders? A systematic review. International Journal of Eating Disorders. 2018;51(3):187–213. [DOI] [PubMed] [Google Scholar]
- 51.Coleman-Jensen A, Rabbitt MP, Gregory CA, Singh A. Household Food Security in the United States in 2017, ERR-256. In: U.S. Department of Agriculture ERS, ed2018. [Google Scholar]
- 52.Rose-Jacobs R, Fiore JG, de Cuba SE, Black M, Cutts DB, Coleman SM, Heeren T, Chilton M, Casey P, Cook J. Children with special health care needs, supplemental security income, and food insecurity. Journal of Developmental & Behavioral Pediatrics. 2016;37(2):140–147. [DOI] [PubMed] [Google Scholar]
- 53.Sutherland R, Katz T, Liu V, Quintano J, Brunner R, Tong CW, Collins CE, Ooi CY. Dietary intake of energy-dense, nutrient-poor and nutrient-dense food sources in children with cystic fibrosis. Journal of Cystic Fibrosis. 2018;17(6):804–810. [DOI] [PubMed] [Google Scholar]
- 54.McDonald CM, Christensen NK, Lingard C, Peet KA, Walker S. Nutrition knowledge and confidence levels of parents of children with cystic fibrosis. ICAN: Infant, Child, & Adolescent Nutrition. 2009;1(6):325–331. [Google Scholar]
- 55.Coleman-Jensen A, Gregory C, Singh A. Household food security in the United States in 2013. USDA-ERS Economic Research Report. 2014(173). [Google Scholar]
- 56.Goss CH, Newsom SA, Schildcrout JS, Sheppard L, Kaufman JD. Effect of ambient air pollution on pulmonary exacerbations and lung function in cystic fibrosis. American journal of respiratory and critical care medicine. 2004;169(7):816–821. [DOI] [PubMed] [Google Scholar]
- 57.Psoter KJ, De Roos AJ, Mayer JD, Kaufman JD, Wakefield J, Rosenfeld M. Fine particulate matter exposure and initial Pseudomonas aeruginosa acquisition in cystic fibrosis. Annals of the American Thoracic Society. 2015;12(3):385–391. [DOI] [PubMed] [Google Scholar]
- 58.Clark LP, Millet DB, Marshall JD. National patterns in environmental injustice and inequality: outdoor NO2 air pollution in the United States. PloS one. 2014;9(4):e94431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grineski SE, Herrera JM, Bulathsinhala P, Staniswalis JG. Is there a Hispanic Health Paradox in sensitivity to air pollution? Hospital admissions for asthma, chronic obstructive pulmonary disease and congestive heart failure associated with NO2 and PM2. 5 in El Paso, TX, 2005–2010. Atmospheric Environment. 2015;119:314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwartz NA, von Glascoe CA, Torres V, Ramos L, Soria-Delgado C. “Where they (live, work and) spray”: Pesticide exposure, childhood asthma and environmental justice among Mexican-American farmworkers. Health & place. 2015;32:83–92. [DOI] [PubMed] [Google Scholar]
- 61.Campbell PW, Parker RA, Roberts BT, Krishnamani M, Phillips JA. Association of poor clinical status and heavy exposure to tobacco smoke in patients with cystic fibrosis who are homozygous for the F508 deletion. The Journal of pediatrics. 1992;120(2):261–264. [DOI] [PubMed] [Google Scholar]
- 62.Collaco JM, Vanscoy L, Bremer L, McDougal K, Blackman SM, Bowers A, Naughton K, Jennings J, Ellen J, Cutting GR. Interactions between secondhand smoke and genes that affect cystic fibrosis lung disease. Jama. 2008;299(4):417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang TW, Asman K, Gentzke AS, Cullen KA, Holder-Hayes E, Reyes-Guzman C, Jamal A, Neff L, King BA. Tobacco product use among adults—United States, 2017. Morbidity and Mortality Weekly Report. 2018;67(44):1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rasmussen JE, Sheridan JT, Polk W, Davies CM, Tarran R. Cigarette smoke-induced Ca2+ release leads to cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction. Journal of Biological Chemistry. 2014;289(11):7671–7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gilreath TD, Leventhal A, Barrington-Trimis JL, Unger JB, Cruz TB, Berhane K, Huh J, Urman R, Wang K, Howland S. Patterns of alternative tobacco product use: emergence of hookah and e-cigarettes as preferred products amongst youth. Journal of Adolescent Health. 2016;58(2):181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hess CA, Olmedo P, Navas-Acien A, Goessler W, Cohen JE, Rule AM. E-cigarettes as a source of toxic and potentially carcinogenic metals. Environmental research. 2017;152:221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. American journal of preventive medicine. 1998;14(4):245–258. [DOI] [PubMed] [Google Scholar]
- 68.Johnson SB, Riley AW, Granger DA, Riis J. The science of early life toxic stress for pediatric practice and advocacy. Pediatrics. 2013;131(2):319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shonkoff JP, Garner AS. Committee on Psychosocial Aspects of Child and Family Health Committee on Early Childhood, Adoption, and Dependent Care Section on Developmental and Behavioral Pediatrics The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232–e246. [DOI] [PubMed] [Google Scholar]
- 70.Chartier MJ, Walker JR, Naimark B. Separate and cumulative effects of adverse childhood experiences in predicting adult health and health care utilization. Child abuse & neglect. 2010;34(6):454–464. [DOI] [PubMed] [Google Scholar]
- 71.Jonson-Reid M, Kohl PL, Drake B. Child and adult outcomes of chronic child maltreatment. Pediatrics. 2012;129(5):839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burke NJ, Hellman JL, Scott BG, Weems CF, Carrion VG. The impact of adverse childhood experiences on an urban pediatric population. Child abuse & neglect,. 2011;35(6):408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lanier P, Jonson-Reid M, Stahlschmidt MJ, Drake B, Constantino J. Child maltreatment and pediatric health outcomes: A longitudinal study of low-income children. Journal of Pediatric Psychology. 2009;35(5):511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hussey JM, Chang JJ, Kotch JB. Child maltreatment in the United States: Prevalence, risk factors, and adolescent health consequences. Pediatrics. 2006;118(3):933–942. [DOI] [PubMed] [Google Scholar]
- 75.Flaherty EG, Thompson R, Dubowitz H, Harvey EM, English DJ, Proctor LJ, Runyan DK. Adverse childhood experiences and child health in early adolescence. JAMA Pediatrics. 2013;167(7):622–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wing R, Gjelsvik A, Nocera M, McQuaid EL. Association between adverse childhood experiences in the home and pediatric asthma. Annals of Allergy, Asthma & Immunology,. 2015;114(5):379–384. [DOI] [PubMed] [Google Scholar]
- 77.Berg KL, Shiu C-S, Feinstein RT, Msall ME, Acharya K. Adverse Childhood Experiences Are Associated with Unmet Healthcare Needs among Children with Autism Spectrum Disorder. The Journal of pediatrics. 2018;202:258–264. [DOI] [PubMed] [Google Scholar]
- 78.McDade TW, Ryan CP, Jones MJ, Hoke MK, Borja J, Miller GE, Kuzawa CW, Kobor MS. Genome‐wide analysis of DNA methylation in relation to socioeconomic status during development and early adulthood. American journal of physical anthropology. 2019. [DOI] [PubMed] [Google Scholar]
- 79.Jongen C, McCalman J, Bainbridge R. Health workforce cultural competency interventions: a systematic scoping review. BMC health services research. 2018;18(1):232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jackson CS, Gracia JN. Addressing health and health-care disparities: the role of a diverse workforce and the social determinants of health. Public Health Reports. 2014;129(1_suppl2):57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hager ER, Quigg AM, Black MM, Coleman SM, Heeren T, Rose-Jacobs R, Cook JT, de Cuba SAE, Casey PH, Chilton M. Development and validity of a 2-item screen to identify families at risk for food insecurity. Pediatrics. 2010;126(1):e26–e32. [DOI] [PubMed] [Google Scholar]
- 82.Frank DA, Casey PH, Black MM, Rose-Jacobs R, Chilton M, Cutts D, March E, Heeren T, Coleman S, de Cuba SE. Cumulative hardship and wellness of low-income, young children: multisite surveillance study. Pediatrics. 2010;125(5):e1115–e1123. [DOI] [PubMed] [Google Scholar]
- 83.Herman D, Afulani P, Coleman-Jensen A, Harrison GG. Food insecurity and cost-related medication underuse among nonelderly adults in a nationally representative sample. American journal of public health. 2015;105(10):e48–e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mabli J, Cohen R, Potter F, Zhao Z. Hunger in America 2010. Mathematica Policy Research. 2010. [Google Scholar]
- 85.McCarthy M “Alarming” rise in popularity of e-cigarettes is seen among US teenagers as use triples in a year. In: British Medical Journal Publishing Group; 2015. [DOI] [PubMed] [Google Scholar]
- 86.Bethell CD, Newacheck P, Hawes E, Halfon N. Adverse childhood experiences: assessing the impact on health and school engagement and the mitigating role of resilience. Health Affairs. 2014;33(12):2106–2115. [DOI] [PubMed] [Google Scholar]
- 87.Bartlett JD, Barto B, Griffin JL, Fraser JG, Hodgdon H, Bodian R. Trauma-informed care in the Massachusetts child trauma project. Child maltreatment. 2016;21(2):101–112. [DOI] [PubMed] [Google Scholar]
- 88.Ortiz R, Sibinga E. The role of mindfulness in reducing the adverse effects of childhood stress and trauma. Children. 2017;4(3):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Policy NIH and Guidelines on The Inclusion of Women and Minorities as Subjects in Clinical Research. https://grants.nih.gov/grants/funding/women_min/guidelines.htm. Accessed 05/08/2019, 2019.