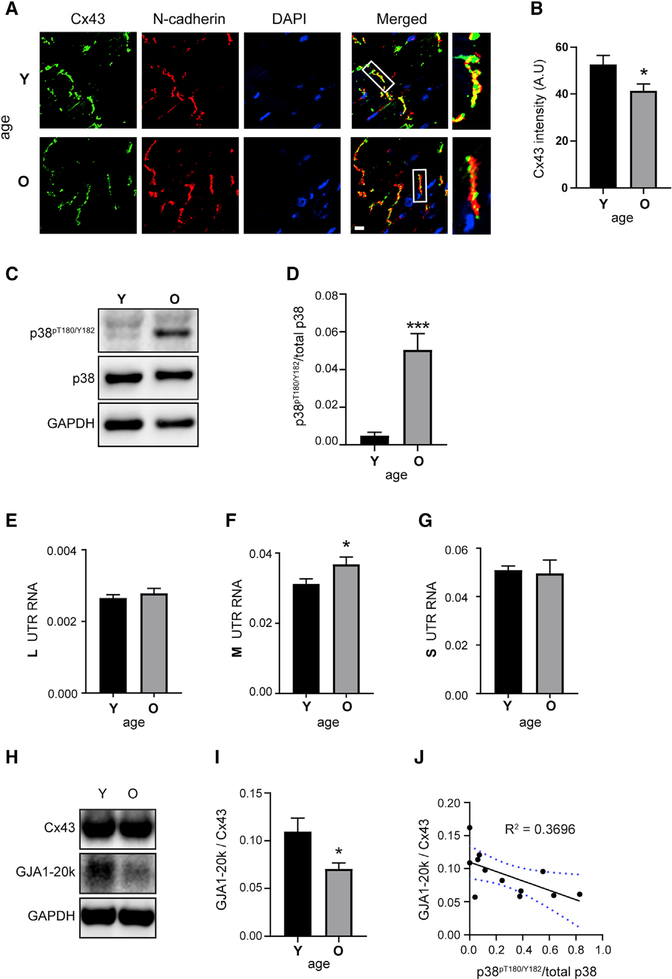
Figure 6. Aging Hearts Have Increased p38, Altered UTRs, and Reduced Internal Translation.
(A) Immunofluorescence (×60) of mouse heart cryosections. Young (Y) = 3.8 months and old (O) = 29 months labeled with an antibody directed against the Cx43 C terminus (green), with intercalated discs labeled with N-cadherin (red) and DAPI DNA stain (blue). Scale bar: 10 μm.
(B) Quantification of average Cx43 fluorescence intensity within N-cadherin-labeled intercalated discs (n = 5 animals, 23 averaged intercalated disc intensities from young and n = 6 animals, 29 averaged intercalated disc intensities from old).
(C) Western blot of mouse heart lysates probed with antibodies directed against phosphorylated p38 (p38pT180/Y182), total p38, and a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) loading control (n = 6 per group).
(D) Quantification of bands from (C).
(E–G) qRT-PCR analysis of L (E), M (F), and S (G) UTR isoforms from mouse heart RNA (n = 6 per group).
(H) Western blot of mouse heart lysates probed with a Cx43 C-terminal antibody to detect full-length Cx43 and internally translated isoform GJA1–20k, and a GAPDH loading control (n = 6 per group).
(I) Quantification of GJA1–20k relative to Cx43 from western blot in (H).
(J) Correlation between GJA1–20k relative to Cx43 and phosphorylated p38pT180/Y182; blue dotted lines represent 95% confidence interval. Statistical analysis performed using the Student’s t test, *p ≤ 0.05, ***p ≤ 0.001.