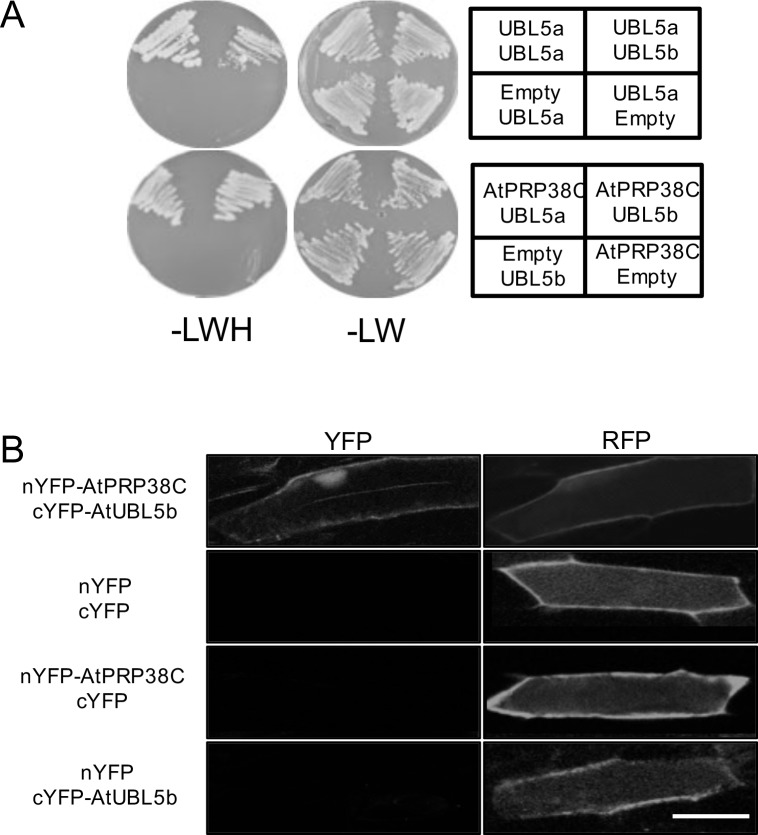
Fig 4. AtUBL5a and AtUBL5b interact with the C-terminal domain of AtPRP38.
(A) Interactions between AtUBL5a, AtUBL5b, and AtPRP38C were detected using a yeast two-hybrid system. The AH109 strain was co-transformed with the constructs indicated, carrying a binding domain and an activation domain, and grown on synthetic drop-out (SD) media lacking leucine and tryptophan (LW) or leucine, tryptophan, and histidine (LWH) with 0.5 mM 3-amino-1,2,4-triazole (3-AT). Yeast containing both vectors could grow on SD-LW. Positive interactions appear as white spots on SD-LWH. (B) BiFC analysis of interactions between AtUBL5b and AtPRP38C. The cDNA constructs (left) were introduced into onion epidermis by particle bombardment, and fluorescence was observed after 1 day. YFP shows the reconstituted YFP fluorescence signal, showing the interaction between AtUBL5b and AtPRP38C in the nucleus. RFP indicates successful introduction of vectors. nYFP and cYFP denote the amino- and carboxyl-terminal halves of YFP, respectively. Bar = 50 μm.