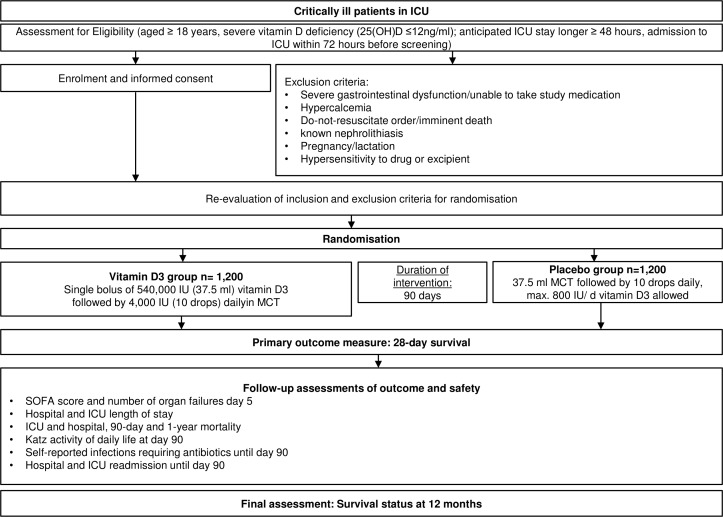
Figure 1.
Trial flow of intervention scheme. We will check eligibility and obtain informed consent* from patients or legally authorised representative/healthcare proxy. After evaluation of exclusion criteria, patients will be randomised in the intervention or placebo group. Primary endpoint is 28-day all-cause mortality. *When informed consent is not possible at time of screening, country-specific alternative strategies for obtaining informed consent are used (ie, in Austria delayed informed consent, in Germany, consent of legal representative, England and Wales consent of relatives or responsible clinician). ICU, intensive care unit; IU, international unit; MCT, median chain triglycerides; SOFA, Sequential Organ Failure Assessment.