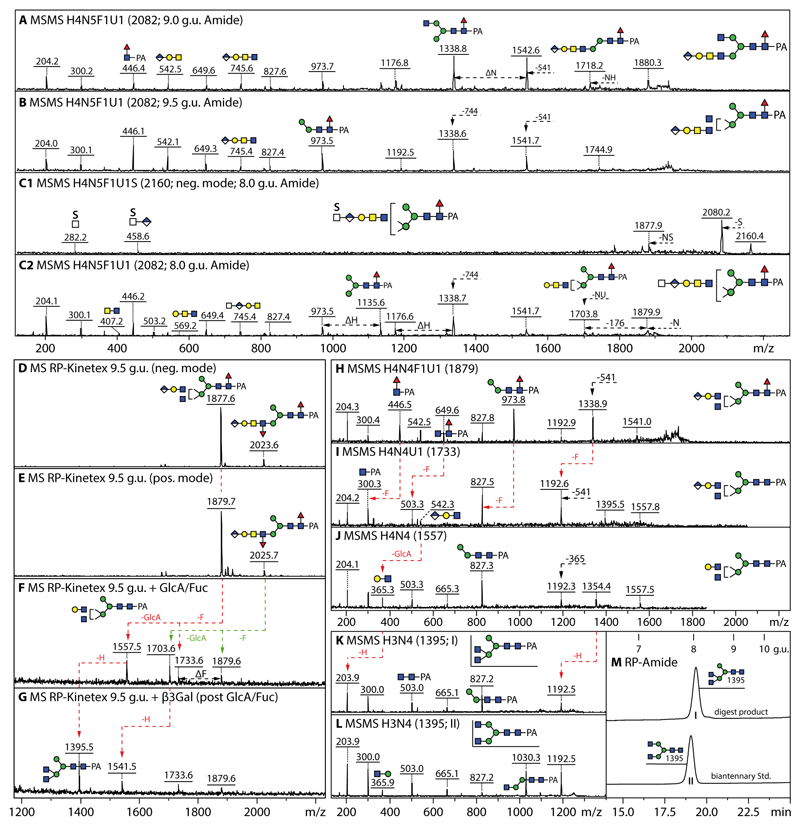
Figure 4. Analysis of example monoglucuronylated biantennary and hybrid honeybee N-glycans.
(A and B) MALDI-TOF MS/MS data of two isomers of HexA1Hex4HexNAc5Fuc1 (m/z 2082, eluting at either 9.0 or 9.5 g.u.) analysed in positive mode. (C1 and C2) MALDI-TOF MS/MS data of HexA1Hex4HexNAc5Fuc1S1 analysed in both negative and positive modes (m/z 2160 and 2082 as [M-H]- and [M-SO3]+), whereby the m/z 458 B-fragment would indicate the presence of a sulphated HexNAcHexA terminal modification, while the underlying structure is revealed by fragmentation of the pseudomolecular ion resulting from ‘in source’ loss of sulphate. Losses of 541 or 744 Da (Hex1HexNAc1-2HexA1) or of NH (HexNAc1Hex1), NU (HexNAc1HexA1) or sulphate (S) from the molecular ion are noted. Compositions are of the form HxNyF1U1S whereby H is hexose, N N-acetylhexosamine, F fucose, U glucuronic acid and S sulphate. (D-G) Negative and positive mode MALDI-TOF MS of the larval anionic 9.5 g.u. fraction, also after subsequent Helix β-glucuronidase/α-fucosidase and Xanthomonas β1,3-galactosidase digestion. (H-J) MS/MS of the m/z 1879 glycan and its glucuronidase/fucosidase digestion products showing shifts in the fragmentation pattern due to loss of core fucose and antennal glucuronic acid. (K and L) Comparison of the MS/MS of the final galactosidase digestion product (I) with that of a standard biantennary N-glycan (II); also the RP-HPLC retention time (M) of the m/z 1395 digestion product is slightly different as that of the biantennary isomer, indicative that the original glycan is a hybrid structure with a β1,2/β1,4-disubstituted α1,3-linked mannose. The tendency for β1,4-GlcNAc to result in later retention times has been previously noted by Tomiya et al (50) and was also observed for Dirofilaria immitis glycans (51).