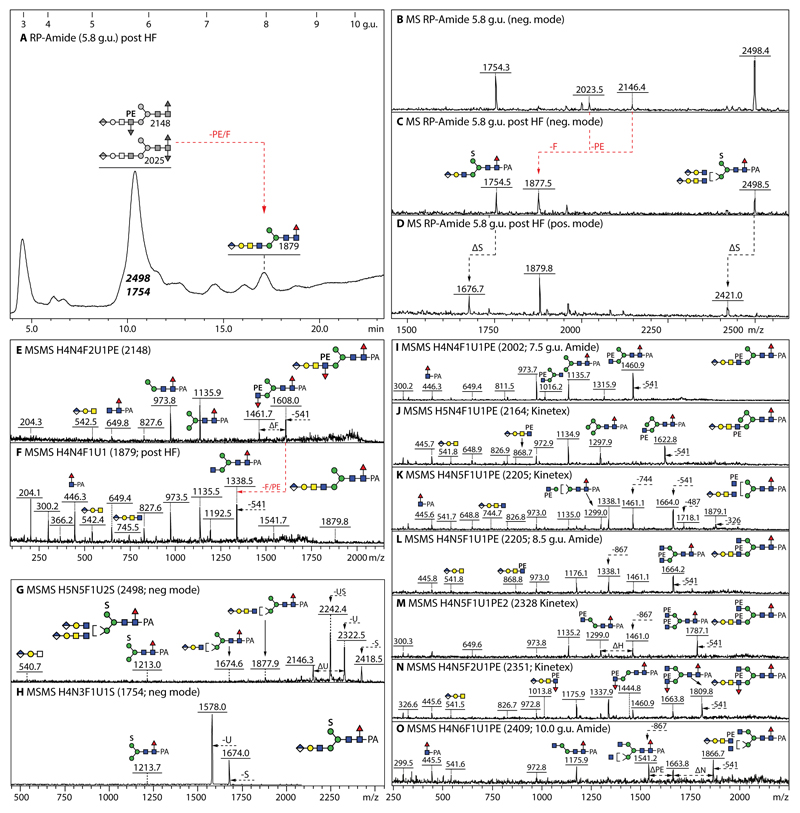
Figure 6. Analysis of example glucuronylated N-glycans modified with fucose, phosphoethanolamine or sulphate residues.
(A-D) RP-HPLC and MALDI-TOF MS of the 5.8 g.u. fraction upon hydrofluoric acid treatment, showing the shift in retention time (+2 g.u.) due to loss of either one core fucose residue from m/z 2025 or of one phosphoethanolamine and an antennal fucose from m/z 2148 resulting in the same m/z 1877/1879 product; the sulphate modifications of the co-eluting m/z 1754 and 2498 glycans are only lost ‘in source’ when performing positive mode MALDI-TOF MS, while the greyscale structures indicate the original elution times of the PE/α3Fuc-containing glycans before HF treatment. (E-H) MALDI-TOF MS/MS of the m/z 2148 glycan, the m/z 1879 product and the two sulphated m/z 1754 and 2498 glycans. (I-O) Example positive mode MALDI-TOF MS of N-glycans carrying one glucuronic acid and one or two phosphoethanolamine residues; phosphoethanolamine-containing B-fragments, e.g., at m/z 868 or 1014 (Hex1HexNAc2HexA1Fuc0-1PE1), are not always observed, but loss of whole or partial antennae results in phosphoethanolamine-containing Y-fragments, e.g., at m/z 1664 or 1460, indicative for substitution of a β1,2-linked GlcNAc by the zwitterionic moiety.