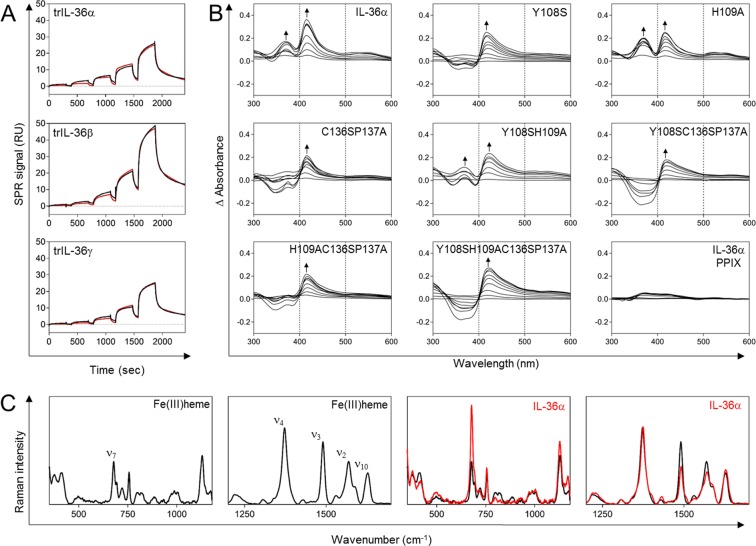
Figure 2.
Spectroscopic studies on heme binding to agonistic IL-36 family members and IL-36α protein mutants. (A) SPR signal (RU) of trIL-36α, β, and γ with five consecutive heme injections of increasing heme concentrations (80 nM to 20 μM). The single-cycle kinetics method was employed (fit is displayed in red). (B) UV/Vis differential spectra of heme-incubated IL-36α and protein mutants. Arrows denote UV/Vis maxima whereas dashed lines at 400 and 500 nm indicate UV/Vis band width in order to illustrate band broadening which was previously found for unspecific heme binding30. (C) Raman spectra of heme (in black), and pentacoordinated wild-type IL-36α (in red) including wavenumber fingerprint region with assignment of prominent normal-mode frequencies ν7 (681 cm−1), ν4 (1374 cm−1), ν3 (1492 cm−1), ν2 (1571 cm−1), and ν10 (1628 cm−1) for heme.