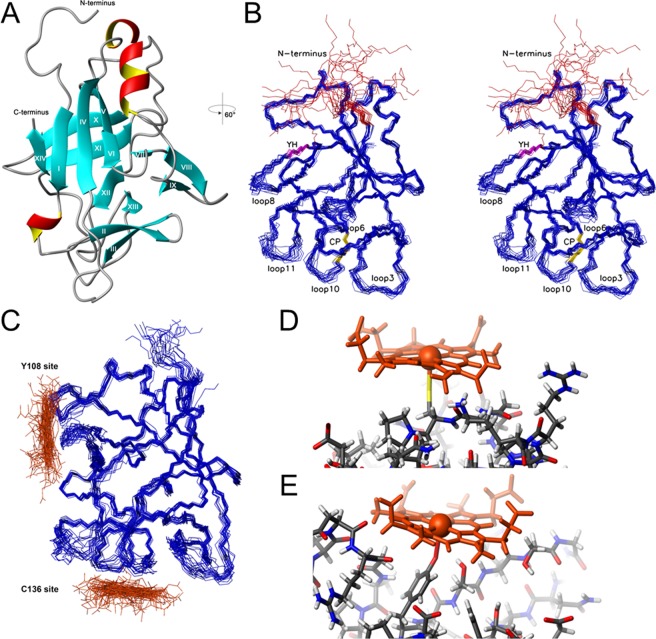
Figure 3.
Structural analysis of IL-36α. (A) NMR structure of IL-36α with numbered β-sheet elements indicated by cyan arrows, α-helical elements in red/yellow and loop regions in grey. (B) Stereoview of the 20 best energy minimized conformers. Flexible loops are indicated and the flexible N-terminal residues, the C136P137, and Y108H109 sites are colored (Met1-Thr9 – red, Cys136Pro137 – gold, Tyr108His109 – magenta). (C–E) Bundle of the IL-36α-heme complex (IL-36α backbone in blue, heme in orange) (C). Detailed view of the heme coordination at Cys136 (D) and Tyr108 (E).