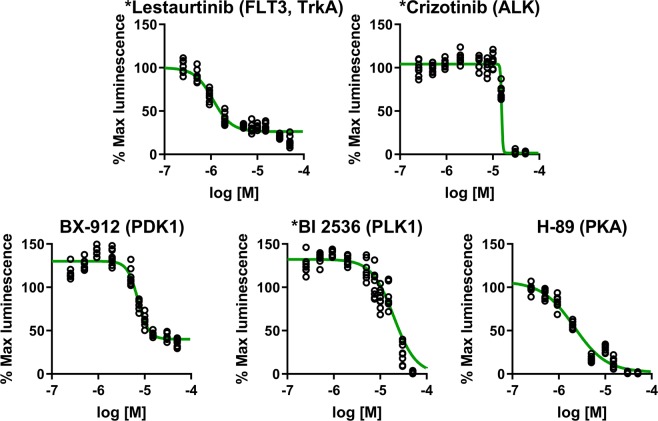
Figure 6.
Second validation of prioritized hits using repurchased compounds. Based on initial dose responses (potency, efficacy, and curve shape) as well as target information, the tyrosine kinase inhibitors Lestaurtinib and Crizotinib, as well as the S/T kinase inhibitors H-89, BX-912, and BI 2536, were repurchased to confirm compound identity and potency. Fresh compounds were tested by 10-point dose responses (range: 0.25 µM–50 µM) in Clone V cells under identical conditions as the primary screening. All compounds demonstrated results similar to the original. While Lestaurtinib, Crizotinib, and H-89 appear to have purely inhibitory effects, BX-912 and BI 2536 act as enhancers at lower concentrations. Estimated IC50: Lestaurtinib, 0.95 µM; Crizotinib, 15.5 µM; H-89, 1.9 µM; BX-912, 6.8 µM; BI 2536, 17.4 µM. *Drug has completed and/or on-going clinical trials, including for PNS or CNS-related cancers.