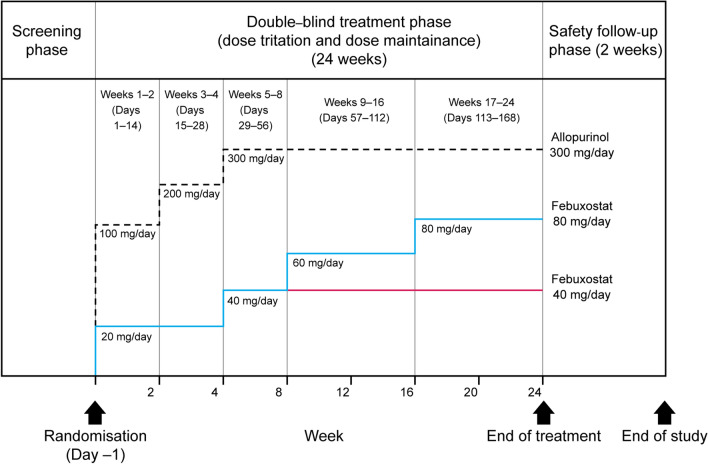
Fig. 1.
Study design and dose-titration scheme for febuxostat 40 mg/day, 80 mg/day, and allopurinol 300 mg/day groups. The study included a screening phase, a double-blind randomized treatment phase (including a dose-titration period and a dose maintenance period, totaling 24 weeks) and a safety follow-up phase (2 weeks). After the screening phase, subjects were randomized into a 24-week treatment phase in which febuxostat and allopurinol were gradually up-titrated to the randomized dose; this was followed-up with a 2-week safety phase. The last visit was an end-of-treatment visit or an early termination visit