Fig. 7.
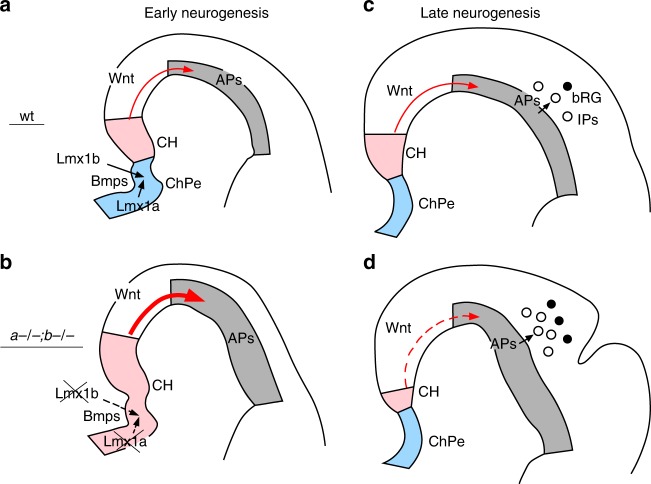
Lmx1a/b-dependent dorsomedial tissue interactions regulate cortical gyrification. a In early wild-type embryos, Lmx1a and Lmx1b are expressed in the telencephalic DMe and adjacent mesenchyme, respectively, and promote expression of secreted Bmps within their respective territories. Appropriate level of Bmp signaling specifies ChPe and CH in the DMe. Wnt3a signaling from the CH (red arrow) promotes proliferation of APs in the cortical VZ. Although we show Wnt signaling by arrow extending through neuroepithelium, our data cannot exclude a possibility that Wnt molecules are delivered to neocortical primordium via CSF. b Simultaneous loss of Lmx1a and 1b reduces Bmp expression in both the mesenchyme and DMe, decreasing the level of Bmp signaling in the DMe. This results in DMe overproliferation and mispatterning, creating an enlarged Wnt3a expression domain at the DM in early Lmx1a−/−;b−/− embryos. The excessive secreted Wnt3a (large red arrow) overactivates canonical Wnt-β-catenin signaling in distal cortical primordium, leading to excessive expansion of APs in Lmx1a−/−;b−/− mutants. c At later developmental stages, in wild-type embryos, Wnt-β-catenin signaling (red arrow) limits production of IPs and bRG from APs29. d By e15.5, normal DMe specification is restored in Lmx1a−/−;b−/− embryos, but enhanced apoptosis reduces the size of CH and DM-derived Wnt signaling (dashed red line). Reduced Wnt signaling allows excessive production of IPs and bRG from an earlier expanded AP population, resulting in cortical gyrification