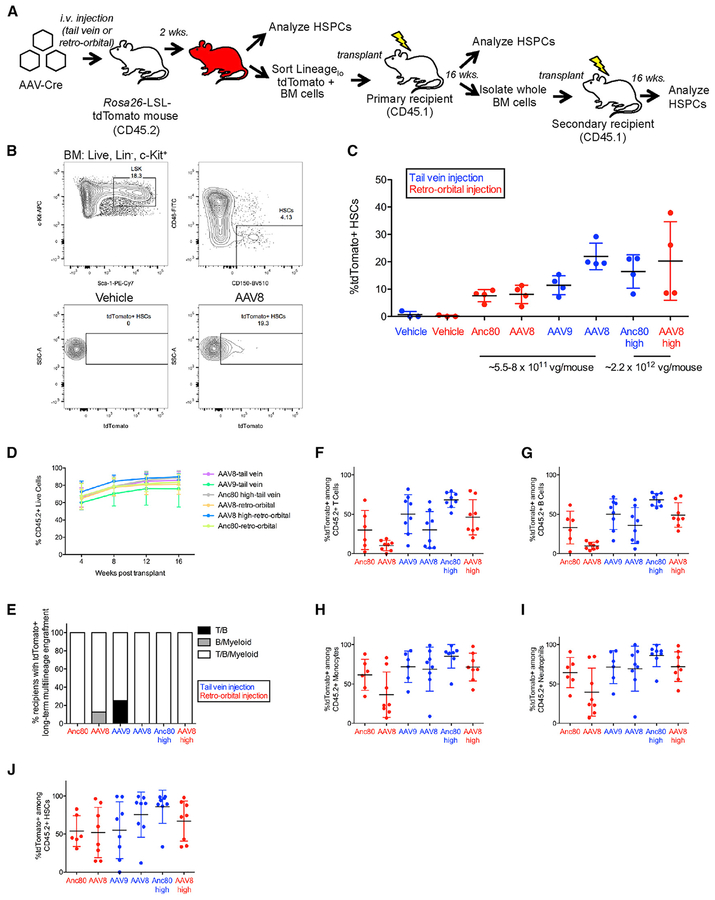
Figure 3. Systemic Injection of AAV-Cre Transduces Functional Hematopoietic Stem Cells.
(A) Experimental design. AAVs encoding Cre recombinase were injected into mdx;Ai9 mice carrying the Rosa26-LSL-tdTomato reporter. i.v., intravenous; LSL, LoxP-STOP-LoxP; HSPCs, hematopoietic stem and progenitor cells.
(B) HSC gating strategy (Lin−Sca-1+c-Kit+CD48−CD150+) from bone marrow (BM) and representative flow cytometric analysis of tdTomato expression within HSCs. LSK: Lin−Sca-1+c-Kit+.
(C) Frequency of HSCs expressing tdTomato. Data points from individual mice are overlaid with mean ± SD. n = 4 mice injected with each AAV serotype per group, n = 3 vehicle-injected mice per group.
(D) Percentage of donor chimerism among live peripheral blood cells of primary recipients at monthly intervals post-transplantation. Data points represent mean ± SD. n = 8 primary recipients per group.
(E) Frequency of primary recipients with tdTomato+ multilineage engraftment within the indicated peripheral blood lineages at 16 weeks post-transplant. For each lineage, tdTomato+ engraftment was scored based on satisfaction of two criteria: >1% CD45.2+ cells and >1% tdTomato+ among CD45.2+ cells.
(F–I) Frequency of tdTomato+ cells among live CD45.2+ peripheral blood (F) T cells, (G) B cells, (H) monocytes, and (I) neutrophils in primary recipients at 16 weeks post-transplantation. For each lineage, only recipients exhibiting >1% CD45.2+ cells within that lineage are shown. Individual data points are shown overlaid with mean ± SD. n = 6–8 primary recipients per group.
(J) Percentage of tdTomato+ HSCs among CD45.2+ HSCs in primary recipients at 6 months post-transplantation Individual data points are shown overlaid with mean ± SD. n = 6–8 primary recipients per group.
vg, viral genomes; Anc80, Anc80L65.
See also Figures S3–S6 and Tables S1 and S2.